Adsorption Behavior of Gold Ions on Nanofiber Webs Containing Protein Polyhedral Crystals
- PMID: 40521473
- PMCID: PMC12163754
- DOI: 10.1021/acsomega.5c01719
Adsorption Behavior of Gold Ions on Nanofiber Webs Containing Protein Polyhedral Crystals
Abstract
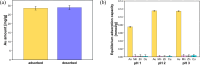
Protein polyhedral crystals (PhCs), with well-defined three-dimensional structures and narrow channels/cavities, have the potential to be utilized as stable biobased adsorbents. However, their intrinsic adsorption abilities for metal ions, including precious metals, remain unclear. In this study, protein PhCs were immobilized in polymer nanofiber (PhC@NF) webs via electrospinning with poly-(ethylene-co-vinyl alcohol) (EVOH). The morphology and adsorption behavior of the PhC@NF web for precious metal ions such as gold (Au) were investigated by scanning electron microscopy and inductively coupled plasma mass spectrometry, respectively. At 25 °C, Au adsorbed only on PhCs and the PhC@NF web exhibited a maximum Au adsorption capacity of 51.7 mg g-1 at pH 1 and a maximum Au adsorption equilibrium constant of 1.02 L mg-1 at pH 3, the highest constant among reported Au ion adsorbents. By contrast, Au reduced by PhCs was deposited as particles on EVOH NF web at 65 °C. The study demonstrates that PhCs are promising materials for efficient recovery of Au from low-concentration Au ion solutions.
© 2025 The Authors. Published by American Chemical Society.
Figures










References
-
- Zhao S., Zhang H., Zhu M., Jiang L., Zheng Y.. Electrical Conductivity of Goldene. Phys. Rev. B. 2024;110(8):085111. doi: 10.1103/PhysRevB.110.085111. - DOI
-
- Marin Rivera R., Elgar C. E., Jacobson B., Feeney A., Prentice P., Ryder K. S., Abbott A. P.. Ultra-Fast Extraction of Metals from a Printed Circuit Board Using High Power Ultrasound in a Calcium Chloride-Based Deep Eutectic Solvent. RSC Sustain. 2024;2(2):403–415. doi: 10.1039/D3SU00147D. - DOI
LinkOut - more resources
Full Text Sources
