Fabrication of Aligned Polyhydroxybutyrate Fibrous Scaffolds via a Touchspinning Apparatus
- PMID: 40521545
- PMCID: PMC12163700
- DOI: 10.1021/acsomega.4c11296
Fabrication of Aligned Polyhydroxybutyrate Fibrous Scaffolds via a Touchspinning Apparatus
Abstract
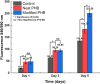
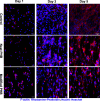
Poly-(3-hydroxybutyrate) (PHB) fibers ranging from nano- to microscale were successfully fabricated using a touchspinning apparatus. The optimization of key spinning parametersincluding solution concentration (5-11% w/v), rotational speed (1300-2100 rpm), and feed rate (5-20 μL/min)enabled the production of aligned fibrous scaffolds. Morphological analysis via field emission scanning electron microscopy (FE-SEM) revealed fiber diameters in the range of 0.831-1.273 μm, which were influenced by spinning conditions. Thermal stability was confirmed using thermogravimetric analysis (TGA), with an onset degradation temperature of ∼290 °C. Differential scanning calorimetry (DSC) showed a melting peak of ∼172 °C and a crystallinity increase from 37.9% in the pellet to 42.5% in fibers of PHB. The scaffolds were functionalized with collagen to enhance bioactivity, and fibroblast (NIH3T3) viability was assessed through alamarBlue and Live/Dead assays. Metabolic activity increased significantly over 5 days (p < 0.05), particularly in collagen-modified scaffolds, confirming excellent cell adhesion and proliferation. Immunofluorescent microscopy demonstrated cell elongation along the fiber axis, indicating scaffold-guided cellular orientation. The results establish the feasibility of touchspun PHB scaffolds for tissue engineering applications, offering a scalable alternative to the conventional electrospinning process.
© 2025 The Authors. Published by American Chemical Society.
Figures







References
-
- Ghimire U., Kandel R., Ko S. W., Adhikari J. R., Kim C. S., Park C. H.. Electrochemical technique to develop surface-controlled polyaniline nano-tulips (PANINTs) on PCL-reinforced chitosan functionalized (CS-f-Fe2O3) scaffolds for stimulating osteoporotic bone regeneration. Int. J. Biol. Macromol. 2024;264:130608. doi: 10.1016/j.ijbiomac.2024.130608. - DOI - PubMed
-
- Kenawy E.-R., Bowlin G. L., Mansfield K., Layman J., Simpson D. G., Sanders E. H., Wnek G. E.. Release of tetracycline hydrochloride from electrospun poly(ethylene-co-vinylacetate), poly(lactic acid), and a blend. J. Controlled Release. 2002;81(1):57–64. doi: 10.1016/S0168-3659(02)00041-X. - DOI - PubMed
LinkOut - more resources
Full Text Sources
