Blood α-Synuclein Separates Parkinson's Disease from Dementia with Lewy Bodies
- PMID: 40521808
- PMCID: PMC12262490
- DOI: 10.1002/ana.27288
Blood α-Synuclein Separates Parkinson's Disease from Dementia with Lewy Bodies
Abstract
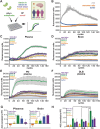
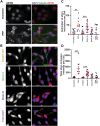
Objective: Aggregation of misfolded α-synuclein (aSyn) within the brain is the pathologic hallmark of Lewy body diseases (LBDs), including Parkinson's disease (PD), and dementia with Lewy bodies (DLB) disease. Although evidence exists for aSyn "strains," conformations with distinct biological properties, biomarkers for PD versus DLB are lacking. Here, we used monoclonal antibodies selective for two different in vitro aSyn species - termed strain A and B - to evaluate human brain tissue, cerebrospinal fluid (CSF), and plasma.
Methods: Using these antibodies, we characterized specific aSyn species in human specimens from neurologically normal individuals and individuals with LBD using enzyme-linked immunosorbent assay (ELISA), Western blot, and immunohistochemistry. We also characterized aSyn species immunoprecipitated from brain lysate or plasma with these antibodies using seed amplification assays (SAAs) and a cellular model.
Results: Surprisingly, levels of strain A and B aSyn species were higher in plasma from individuals with PD compared to DLB in 2 independent cohorts. Lower levels of plasma aSyn strain A species predicted a faster rate of cognitive decline in individuals with PD. Furthermore, strain A and strain B aSyn species were undetectable in CSF, and their levels in brain versus plasma did not correlate. Moreover, plasma aSyn species isolated by aSyn strain antibodies could template aSyn fibrillization, and they could seed formation of aSyn inclusions in cells.
Interpretation: Our findings suggest that circulating plasma aSyn strains may impact LBD clinical presentation, particularly cognition. The enrichment of these aSyn species in plasma but not CSF also suggests a potential source outside the brain. ANN NEUROL 2025;98:682-698.
© 2025 The Author(s). Annals of Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
T.F.T. has received consulting fees and honoraria from Sanofi Genzyme, Bial, and the Parkinson Foundation. The following authors declare that they have no competing interests: G.T.K., S.A., R.S.A., J.F.M., A.C.P., D.J.I., R.T.S., R.D.R., K.D., and V.L.
Figures





References
MeSH terms
Substances
Grants and funding
- Chan Zuckerberg Initiative Neurodegeneration Challenge Network
- Weston Institute
- P01 AG084497/AG/NIA NIH HHS/United States
- R01NS082265/NH/NIH HHS/United States
- K08 NS093127/NS/NINDS NIH HHS/United States
- Lewy Body Dementia Association Research Center of Excellence Program
- R01 NS115139/NS/NINDS NIH HHS/United States
- U19 AG062418/AG/NIA NIH HHS/United States
- Michael J. Fox Foundation/Alzheimer's Association
- P30 AG072979/AG/NIA NIH HHS/United States
- R01 NS082265/NS/NINDS NIH HHS/United States
- K23 NS114167/NS/NINDS NIH HHS/United States
- American Heart Association/Allen Brain Health Initiative
- Parker Family Chair
- K23-NS114167/NH/NIH HHS/United States
- Parkinson Foundation
- P01AG066597/NH/NIH HHS/United States
- P50 NS053488/NS/NINDS NIH HHS/United States
- R01NS115139/NH/NIH HHS/United States
- P30 ES013508/ES/NIEHS NIH HHS/United States
- Penn Institute on Aging
- T32 NS091008/NS/NINDS NIH HHS/United States
- P01 AG066597/AG/NIA NIH HHS/United States
- R37 NS115139/NS/NINDS NIH HHS/United States
- P50 NS053488/NH/NIH HHS/United States
- K08NS093127/NH/NIH HHS/United States
- T32 T32-NS091008-06/NH/NIH HHS/United States
- P30AG072979/NH/NIH HHS/United States
- U19 AG062418/NH/NIH HHS/United States
- R01NS115139/NH/NIH HHS/United States
- R01NS082265/NH/NIH HHS/United States
- P30AG072979/NH/NIH HHS/United States
- P01AG066597/NH/NIH HHS/United States
- U19 AG062418/NH/NIH HHS/United States
- P50 NS053488/NH/NIH HHS/United States
- T32 T32-NS091008-06/NH/NIH HHS/United States
- K08NS093127/NH/NIH HHS/United States
- K23-NS114167/NH/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

