Rate of brain aging associates with future executive function in Asian children and older adults
- PMID: 40522287
- PMCID: PMC12169851
- DOI: 10.7554/eLife.97036
Rate of brain aging associates with future executive function in Asian children and older adults
Abstract
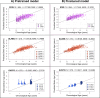
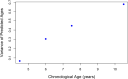
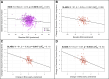
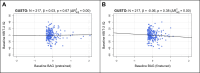
Brain age has emerged as a powerful tool to understand neuroanatomical aging and its link to health outcomes like cognition. However, there remains a lack of studies investigating the rate of brain aging and its relationship to cognition. Furthermore, most brain age models are trained and tested on cross-sectional data from primarily Caucasian, adult participants. It is thus unclear how well these models generalize to non-Caucasian participants, especially children. Here, we tested a previously published deep learning model on Singaporean elderly participants (55-88 years old) and children (4-11 years old). We found that the model directly generalized to the elderly participants, but model finetuning was necessary for children. After finetuning, we found that the rate of change in brain age gap was associated with future executive function performance in both elderly participants and children. We further found that lateral ventricles and frontal areas contributed to brain age prediction in elderly participants, while white matter and posterior brain regions were more important in predicting brain age of children. Taken together, our results suggest that there is potential for generalizing brain age models to diverse populations. Moreover, the longitudinal change in brain age gap reflects developing and aging processes in the brain, relating to future cognitive function.
Keywords: T1-weighted MRI; aging; brain age; development; human; longitudinal; machine learning; neuroscience.
© 2024, Cheng et al.
Conflict of interest statement
SC, WY, KN, XQ, SL, TT, KN, RL, SH, CC, AT, EL, PG, CC, YC, MM, MC, BY No competing interests declared, JZ Reviewing editor, eLife
Figures





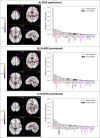


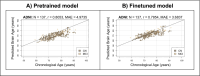
Update of
- doi: 10.1101/2023.11.27.568184
- doi: 10.7554/eLife.97036.1
- doi: 10.7554/eLife.97036.2
Similar articles
-
Brain activations elicited during task-switching generalize beyond the task: A partial least squares correlation approach to combine fMRI signals and cognition.Hum Brain Mapp. 2024 Aug 1;45(11):e26804. doi: 10.1002/hbm.26804. Hum Brain Mapp. 2024. PMID: 39126346 Free PMC article.
-
Lobar microbleeds are associated with a decline in executive functioning in older adults.Cerebrovasc Dis. 2014;38(5):377-83. doi: 10.1159/000368998. Epub 2014 Nov 25. Cerebrovasc Dis. 2014. PMID: 25427958 Free PMC article.
-
White matter in aging and cognition: a cross-sectional study of microstructure in adults aged eighteen to eighty-three.Dev Neuropsychol. 2010;35(3):257-77. doi: 10.1080/87565641003696775. Dev Neuropsychol. 2010. PMID: 20446132 Free PMC article.
-
Cognitive and neural plasticity in old age: A systematic review of evidence from executive functions cognitive training.Ageing Res Rev. 2019 Aug;53:100912. doi: 10.1016/j.arr.2019.100912. Epub 2019 May 30. Ageing Res Rev. 2019. PMID: 31154013
-
A review of the brain structure correlates of successful cognitive aging.J Neuropsychiatry Clin Neurosci. 2011 Winter;23(1):6-15. doi: 10.1176/jnp.23.1.jnp6. J Neuropsychiatry Clin Neurosci. 2011. PMID: 21304134 Free PMC article. Review.
Cited by
-
Mind the Gap: Does Brain Age Improve Alzheimer's Disease Prediction?bioRxiv [Preprint]. 2025 Jun 1:2024.11.16.623903. doi: 10.1101/2024.11.16.623903. bioRxiv. 2025. Update in: Hum Brain Mapp. 2025 Aug 15;46(12):e70276. doi: 10.1002/hbm.70276. PMID: 39605400 Free PMC article. Updated. Preprint.
-
Mind the Gap: Does Brain Age Improve Alzheimer's Disease Prediction?Hum Brain Mapp. 2025 Aug 15;46(12):e70276. doi: 10.1002/hbm.70276. Hum Brain Mapp. 2025. PMID: 40820870 Free PMC article.
References
-
- Abadi M, Agarwal A, Barham P. TensorFlow: Large-scale machine learning on heterogeneous distributed systems. arXiv. 2015 doi: 10.48550/arXiv.1603.04467. - DOI
-
- Avants B, Tustison NJ, Song G. Advanced Normalization Tools: V1.0. The Insight Journal. 2008 doi: 10.54294/uvnhin. - DOI
-
- Bagautdinova J, Bourque J, Sydnor VJ, Cieslak M, Alexander-Bloch AF, Bertolero MA, Cook PA, Gur RC, Gur RE, Larsen B, Moore TM, Radhakrishnan H, Roalf DR, Shinohara RT, Tapera TM, Zhao C, Sotiras A, Davatzikos C, Satterthwaite TD. Development of White Matter Fiber Covariance Networks Supports Executive Function in Youth. bioRxiv. 2023 doi: 10.1101/2023.02.09.527696. - DOI - PMC - PubMed
-
- Bashyam VM, Erus G, Doshi J, Habes M, Nasrallah I, Truelove-Hill M, Srinivasan D, Mamourian L, Pomponio R, Fan Y, Launer LJ, Masters CL, Maruff P, Zhuo C, Völzke H, Johnson SC, Fripp J, Koutsouleris N, Satterthwaite TD, Wolf D, Gur RE, Gur RC, Morris J, Albert MS, Grabe HJ, Resnick S, Bryan RN, Wolk DA, Shou H, Davatzikos C. MRI signatures of brain age and disease over the lifespan based on a deep brain network and 14 468 individuals worldwide. Brain. 2020;143:2312–2324. doi: 10.1093/brain/awaa160. - DOI - PMC - PubMed
MeSH terms
Grants and funding
- NMRC/OFLCG19May-0035/Singapore National Medical Research Council
- NMRC/CIRG/1485/2018/Singapore National Medical Research Council
- NMRC/CSA-SI/0007/2016/Singapore National Medical Research Council
- NMRC/MOH-00707-01/Singapore National Medical Research Council
- NMRC/CG/435 M009/2017-NUH/NUHS/Singapore National Medical Research Council
- CIRG21nov-0007/Singapore National Medical Research Council
- HLCA23Feb-0004/Singapore National Medical Research Council
- A20G8b0102/Singapore A*STAR
- MOE-T2EP40120-0007/Singapore Ministry of Education
- MOE-T2EP2-0223-0025/Singapore Ministry of Education
- MOE-T2EP20220-0001/Singapore Ministry of Education
- Yong Loo Lin School of Medicine Research Core Funding/National University of Singapore
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

