Malformations of cortical development: Embryology and epilepsy
- PMID: 40522308
- PMCID: PMC12605774
- DOI: 10.1111/epi.18501
Malformations of cortical development: Embryology and epilepsy
Abstract
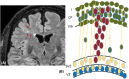
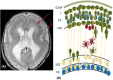
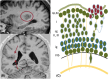
One in seven patients with focal epilepsy has a malformation of cortical development (MCD) as underlying cause. Understanding normal cortical development combined with knowledge of where, when, and what goes wrong in different types of MCD provides insight into the mechanisms of epileptogenesis. Three different steps can be distinguished in the development of the neocortex: proliferation, migration, and organization. These three steps occur at different locations, partly overlapping in time. In this review, we illustrate and correlate normal embryology to the most common MCDs in epilepsy, namely, focal cortical dysplasia, heterotopia, and polymicrogyria, with discriminating imaging findings and clinical implications. By integrating current literature on embryology and imaging findings, we aim to provide insight into classification of cortical malformations and the consequences for workup and treatment. Illustrations of normal cortical embryology and early fetal development are supplemented with magnetic resonance images from our tertiary epilepsy center showing the three most frequently encountered malformations: focal cortical dysplasia (approximately half of identified MCDs at our center, consistent with literature), heterotopia (one third), and polymicrogyria (approximately 10%).
Keywords: focal cortical dysplasia; heterotopia; malformations of cortical development; polymicrogyria.
© 2025 The Author(s). Epilepsia published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
Neither of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures






References
-
- Müller F, O'Rahilly R. The first appearance of the future cerebral hemispheres in the human embryo at stage 14. Anat Embryol. 1988;177(6):495–511. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

