Intraocular inflammation following intravitreal injections of anti-vascular endothelial growth factor drugs
- PMID: 40522452
- PMCID: PMC12583367
- DOI: 10.1007/s00417-025-06875-w
Intraocular inflammation following intravitreal injections of anti-vascular endothelial growth factor drugs
Abstract
Purpose: To describe cases of infectious and non-infectious intraocular inflammation (NI-IOI) associated with intravitreal injections (IVI) of anti-vascular endothelial growth factor (anti-VEGF) drugs in the largest tertiary center with the highest number of IVI in Portugal.
Methods: Prospective observational study including all patients diagnosed with infectious endophthalmitis (IE) or NI-IOI after IVI of different anti-VEGF drugs in a total of 83,145 IVI between 2018 and 2023. The most frequent indications for treatment were macular neovascularization or macular edema from different etiologies.
Results: Twenty-six eyes developed IE (mean incidence 0.031%) after IVI and 24 eyes were diagnosed with NI-IOI (mean incidence 0.028%), including anterior plus intermediate uveitis (n = 18), only vitritis (n = 4) and retinal vasculitis (n = 2). Regarding NI-IOI, eyes were under treatment with aflibercept (n = 12), bevacizumab (n = 11) and faricimab (n = 1). The most common initial presentation was a painless decrease in visual acuity (VA) at a mean of 8 days [1-20] after IVI in patients with NI-IOI versus red eye, pain and blurry vision, at a mean of 3 days [1-9] after IVI in the IE group. There was a significant improvement in VA between the NI-IOI diagnosis and final visits (35 ± 25 vs. 49 ± 24 ETDRS letters) in the NI-IOI group (p = 0.022), an improvement that occurred over a mean of almost 2 months. There were no significant differences between VA at the time of diagnosis and after treatment (25 ± 28 vs. 26 ± 25 ETDRS letters) in the IE group (p = 0.801).
Conclusion: NI-IOI represents an emergent treatment-related adverse event, that can be reversible when adequately managed, contrasting with the sight-threatening severe cases of IE. We emphasize the importance of maintaining active surveillance in patients under intravitreal therapy.
Keywords: Infectious endophthalmitis; Intraocular inflammation; Intravitreal therapy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Compliance with ethical standards: The study was approved by the Institutional Ethics Review Board of Local Health Unit of São João, Porto, Portugal. The protocol conformed with the canons of the Declaration of Helsinki for research involving human participants, as well as the European Union’s General Data Protection Regulation. Informed consent was waived in view of the prospective nature of the study. This article was redacted according to the recommendations of The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. Conflict of interest: The authors have no relevant financial or non-financial interests to disclose.
Figures
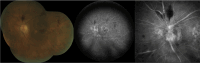

References
-
- Patil NS, Dhoot AS, Popovic MM, Kertes PJ, Muni RH (2022) Risk of intraocular inflammation after injection of antivascular endothelial growth factor agents: a meta-analysis. Retina 42(11):2134–2142 - PubMed
-
- Schwartz SG, Flynn HW Jr, Emerson GG, Choudhry N, Ferrone PJ, Goldberg RA et al (2019) Distinguishing between infectious endophthalmitis and noninfectious inflammation following intravitreal anti-VEGF injection. J VitreoRetinal Dis 3(1):42–44
-
- Fine HF, Despotidis GD, Prenner JL (2015) Ocular inflammation associated with antivascular endothelial growth factor treatment. Curr Opin Ophthalmol 26(3):184–187 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

