Comparative Effectiveness of Fumarates Versus Sphingosine-1-Phosphate Receptor Modulators in Black Patients with Multiple Sclerosis
- PMID: 40522622
- PMCID: PMC12255590
- DOI: 10.1007/s40120-025-00774-2
Comparative Effectiveness of Fumarates Versus Sphingosine-1-Phosphate Receptor Modulators in Black Patients with Multiple Sclerosis
Abstract
Introduction: Multiple sclerosis (MS) is a heterogeneous disease that disproportionately impacts Black people with MS (PwMS), who experience more severe disease and higher relapse rates compared with non-Black populations. Despite widespread use of fumarates and sphingosine-1-phosphate (S1P) receptor modulators as oral disease-modifying therapies (DMTs) for relapsing MS, their comparative effectiveness in Black PwMS has not been studied. This study aims to help address this gap using real-world claims data.
Methods: This retrospective analysis using the Komodo Health Claims Database included Black PwMS. Patients were aged 18-64 years with ≥ 1 claim for MS diagnosis (International Classification of Diseases, Tenth Revision, Clinical Modification code G35) and ≥ 1 prescription claim for fumarates (dimethyl fumarate or diroximel fumarate) or an S1P receptor modulator (fingolimod, siponimod, ozanimod, or ponesimod) between January 2017 and April 2023. Outcomes included annualized relapse rate (ARR) and time to first relapse. Propensity score matching (2:1) and inverse probability weighting were used to balance baseline characteristics. Relapse events were identified using a claims-based algorithm.
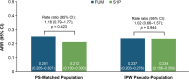
Results: The analysis included 1664 Black PwMS (1231 and 433 in fumarate and S1P treatment arms, respectively). Post-index ARRs were comparable between groups (rate ratio [RR] 1.18, p = 0.423). Kaplan-Meier analyses showed similar relapse-free proportions at 24 months (72.6% and 74.7% in fumerate and S1P populations, respectively; p = 0.152). These findings were consistent in both the propensity score-matched and inverse probability weighted populations.
Conclusions: This real-world, claims-based analysis demonstrates that fumarates and S1P receptor modulators have similar effectiveness in reducing relapses among Black PwMS, with > 72% of patients in both treatment groups remaining relapse-free at 24 months. Given the underrepresentation of Black patients in MS clinical trials, these results provide valuable real-world evidence to guide treatment decisions for this population.
Keywords: African American populations; Dimethyl fumarate; Diroximel fumarate; Effectiveness; Multiple sclerosis; Sphingosine-1-phosphate.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflicts of Interest: Sophia Woodson served on scientific advisory or data safety monitoring boards for MSSA and Sanofi, and served on speaker bureaus for Biogen, Bristol Myers Squibb, and Genentech. Edward J Gettings, Jong-Mi Lee, and Aljoeson Walker declare that they have no competing interests. Chu-Yueh Guo has received consulting fees from Genentech and Horizon. Sylvia Klineova has received consulting fees from Amgen and TG Therapeutics and for contracted research for Biogen, and served on speakers bureaus for Alexion and Biogen. Rebecca S Romero has received consulting fees from Alexion, EMD Serono, Genentech, Horizon, and TG Therapeutics. Nicholas Belviso, Sai L Shankar, Jason P Mendoza, Boyang Bian, James B Lewin, and Kinyee Fong are all employees of Biogen and may hold stock in the company. Ethical Approval: This study was a retrospective analysis of de-identified data from the Komodo Health Sentinel database and did not involve any new studies with human or animal subjects. As such, ethical approval or informed consent was not required.
Figures



References
-
- MS International Federation. Atlas of MS. 2023. https://www.atlasofms.org/map/united-states-of-america/epidemiology/numb.... Accessed 25 Sept 2024.
-
- US Census Bureau. QuickFacts. 2023. https://www.census.gov/quickfacts/fact/table/US/RHI225222. Accessed 25 Sept 2024.
-
- Cipriani VP, Klein S. Clinical characteristics of multiple sclerosis in African-Americans. Curr Neurol Neurosci Rep. 2019;19(11):87. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

