Clonal integration and Bacillus subtilis modulate Glechoma longituba performance and soil microbial communities
- PMID: 40522963
- PMCID: PMC12169573
- DOI: 10.1371/journal.pone.0325605
Clonal integration and Bacillus subtilis modulate Glechoma longituba performance and soil microbial communities
Abstract
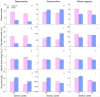
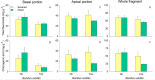
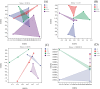
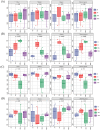
Many medicinal plants exhibit clonality, but the impact of clonal integration and its interaction with exogenous microbial agents on these plants remains unknown. In order to investigate this, we conducted a greenhouse experiment using Glechoma longituba, a common clonal medicinal herb. Pairs of connected ramets were grown in the two adjacent pots, with one pot containing basal (relatively older) ramets treated with or without Bacillus subtilis agent and the other pot containing apical (relatively younger) ramets without B. subtilis agent treatment, the connection between basal and apical ramets were either left intact or severed. Clonal integration reduced the growth of basal ramets, but increased the apical ramet growth. B. subtilis agent primarily affected the root-shoot ratio of both basal and apical ramets as well as the whole fragments. Furthermore, it exhibited a significant interaction with clonal integration in affecting the root-shoot ratio of basal ramets and whole plant fragments. Addition of B. subtilis reduced the content of total flavonoids and chlorogenic acid in basal portions and chlorogenic acid at the whole fragment level. Clonal integration and B. subtilis agent significantly changed the composition of soil fungal communities of basal portions and bacterial communities of apical portions. The fungal composition of basal portions responded reciprocally to clonal integration and B. subtilis, with a significant increase in the relative abundance of Basidiomycota and a decrease in Ascomycota under clonal integration, whereas the effect of B. subtilis was opposite. B. subtilis significantly increased fungal diversity in basal portions while decreasing bacterial diversity in apical portions under clonal integration. However, neither clonal integration nor B. subtilis has showed a positive effect on the overall growth and quality of G. longituba. These findings provide valuable insights into its role in scientific cultivation and management of the clonal medicinal plants in the practical production.
Copyright: © 2025 Zhao et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Effects of clonal integration and nutrient availability on the growth of Glechoma longituba under heterogenous light conditions.Front Plant Sci. 2023 Aug 15;14:1182068. doi: 10.3389/fpls.2023.1182068. eCollection 2023. Front Plant Sci. 2023. PMID: 37649995 Free PMC article.
-
Clonal integration ameliorates the carbon accumulation capacity of a stoloniferous herb, Glechoma longituba, growing in heterogenous light conditions by facilitating nitrogen assimilation in the rhizosphere.Ann Bot. 2015 Jan;115(1):127-36. doi: 10.1093/aob/mcu207. Epub 2014 Nov 26. Ann Bot. 2015. PMID: 25429006 Free PMC article.
-
Partial mechanical stimulation facilitates the growth of the rhizomatous plant Leymus secalinus: modulation by clonal integration.Ann Bot. 2011 Apr;107(4):693-7. doi: 10.1093/aob/mcr012. Epub 2011 Feb 7. Ann Bot. 2011. PMID: 21303785 Free PMC article.
-
Capacity for clonal integration in introduced versus native clones of the invasive plant Hydrocotyle vulgaris.Sci Total Environ. 2020 Nov 25;745:141056. doi: 10.1016/j.scitotenv.2020.141056. Epub 2020 Jul 20. Sci Total Environ. 2020. PMID: 32717606
-
Bacillus subtilis impact on plant growth, soil health and environment: Dr. Jekyll and Mr. Hyde.J Appl Microbiol. 2022 May;132(5):3543-3562. doi: 10.1111/jam.15480. Epub 2022 Mar 6. J Appl Microbiol. 2022. PMID: 35137494 Review.
References
-
- Caraco T, Kelly CK. On the adaptive value of physiological integraton in colonal plants. Ecology. 1991;72(1):81–93. doi: 10.2307/1938904 - DOI
-
- Alpert P. Nutrient sharing in natural clonal fragments of Fragaria chiloensis. J Ecol. 1996;84(3):395. doi: 10.2307/2261201 - DOI
-
- Cao X-X, Xue W, Lei N-F, Yu F-H. Effects of clonal integration on foraging behavior of three clonal plants in heterogeneous soil environments. Forests. 2022;13(5):696. doi: 10.3390/f13050696 - DOI
-
- Chen J-S, Lei N-F, Dong M. Clonal integration improves the tolerance of Carex praeclara to sand burial by compensatory response. Acta Oecol. 2010;36(1):23–8. doi: 10.1016/j.actao.2009.09.006 - DOI
MeSH terms
LinkOut - more resources
Full Text Sources

