Physicochemical quality assessment of four asparaginases
- PMID: 40522975
- PMCID: PMC12169553
- DOI: 10.1371/journal.pone.0326106
Physicochemical quality assessment of four asparaginases
Abstract
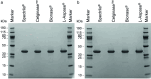
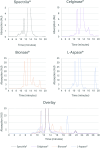
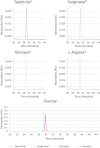
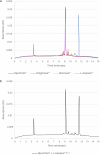
L-Asparaginases (ASNases) are used for the treatment of acute lymphoblastic leukaemia. There are reports of quality problems for some therapeutic asparaginase products, especially those manufactured in middle-income countries. These products may exhibit decreased potency and/or decreased specific activity, or an elevated level of impurities such as host cell proteins. In this study, four different ASNase preparations that were not modified with polyethylene glycol were compared in detail regarding their quality: Spectrila®, Celginase™, Bionase®, and L-Aspase®. Samples were analyzed for protein content, impurities, and enzyme activity. Various chromatographic methods as well as mass spectrometry were used to assess purity and identity. Sample protein content, host cell protein, and enzyme activity showed some results that were out of target range for Celginase™ and Bionase®. These ASNase preparations also showed detectable levels of endotoxins. In gel electrophoresis, additional bands were found for Bionase®. Size exclusion chromatography showed increased high and low molecular weight species for Bionase® and L-Aspase®, and reversed-phase chromatography showed increased hydrophilic and hydrophobic species for Bionase®. In capillary zone electrophoresis, increased retention time for L-Aspase® and increased levels of charge variants for Bionase®, Celginase™, and L-Aspase® were seen. ASNase quality standards are crucial to ensure patient safety and product efficacy, as decreased potency and specific activity may affect efficacy in acute lymphoblastic leukaemia treatment, and increased impurities may affect immunogenicity. Out of four ASNase preparations tested in this study, only Spectrila® did not raise any quality concerns. The other three products exhibited quality problems, rendering them unsuitable according to established quality requirements defined in European and US guidelines for pharmaceutical development of parenteral drug products.
Copyright: © 2025 Radtke et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
Spectrila® is a pharmaceutical product developed and marketed by medac GmbH in several countries including the European Union. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- Mondelaers V, Suciu S, De Moerloose B, Ferster A, Mazingue F, Plat G, et al. Prolonged versus standard native E. coli asparaginase therapy in childhood acute lymphoblastic leukemia and non-Hodgkin lymphoma: final results of the EORTC-CLG randomized phase III trial 58951. Haematologica. 2017;102(10):1727–38. doi: 10.3324/haematol.2017.165845 - DOI - PMC - PubMed
-
- Pieters R, Appel I, Kuehnel H-J, Tetzlaff-Fohr I, Pichlmeier U, van der Vaart I, et al. Pharmacokinetics, pharmacodynamics, efficacy, and safety of a new recombinant asparaginase preparation in children with previously untreated acute lymphoblastic leukemia: a randomized phase 2 clinical trial. Blood. 2008;112(13):4832–8. doi: 10.1182/blood-2008-04-149443 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

