Consensus recommendations for hyperpolarized [1-13C]pyruvate MRI multi-center human studies
- PMID: 40523079
- PMCID: PMC12236423
- DOI: 10.1002/mrm.30570
Consensus recommendations for hyperpolarized [1-13C]pyruvate MRI multi-center human studies
Abstract
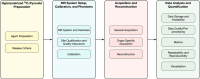
MRI of hyperpolarized (HP) [1-13C]pyruvate allows in vivo assessment of metabolism and has translated into human studies across diseases at 15 centers worldwide. To determine consensus on best practice for multi-center studies for development of clinical applications. This paper presents the results of a two-round formal consensus building exercise carried out by experts with HP [1-13C]pyruvate human study experience. Twenty-nine participants from 13 sites brought together expertise in pharmacy methods, MR physics, translational imaging, and data analysis with the goal of providing recommendations and best practice statements on conduct of multi-center human studies of HP [1-13C]pyruvate MRI. Overall, the group reached consensus on approximately two-thirds of 246 statements in the questionnaire, covering HP 13C-pyruvate preparation; MRI system setup, calibration, and phantoms; acquisition and reconstruction; and data analysis and quantification. Consensus was present across categories. Examples include: (i) Different HP pyruvate preparation methods could be used in human studies, but the same release criteria have to be followed; (ii) site qualification and quality assurance must be performed with phantoms and the same field strength must be used, but the rest of the system setup and calibration methods could be determined by individual sites; (iii) the same pulse sequence and reconstruction methods were preferable, but the exact choice should be governed by the anatomical target; (iv) normalized metabolite area-under-curve values and metabolite area under curve were the preferred metabolism metrics. The consensus proces revealed that HP[1-13C] pyruvate MRI as a technology has progressed sufficiently to plan multi-center studies. The work confirmed areas of consensus for multi-center study conduct and identified where further research is required to ascertain best practice.
© 2025 The Author(s). Magnetic Resonance in Medicine published by Wiley Periodicals LLC on behalf of International Society for Magnetic Resonance in Medicine.
Conflict of interest statement
Figures
Update of
-
Consensus Recommendations for Hyperpolarized [1-13C]pyruvate MRI Multi-center Human Studies.ArXiv [Preprint]. 2025 Apr 29:arXiv:2504.20440v1. ArXiv. 2025. Update in: Magn Reson Med. 2025 Oct;94(4):1386-1400. doi: 10.1002/mrm.30570. PMID: 40342865 Free PMC article. Updated. Preprint.
References
-
- Hubbard Cristinacce PL, Markus JE, Punwani S, et al. Steps on the path to clinical translation: a workshop by the British and Irish chapter of the ISMRM. Magn Reson Med. 2023;90:1130‐1136. - PubMed
-
- Fitch K, Bernstein SJ, Aguilar MD, et al. The RAND/UCLA Appropriateness Method User's Manual. RAND; 2001.
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA008748/CA/NCI NIH HHS/United States
- R01 CA262630/CA/NCI NIH HHS/United States
- NIH P41EB013598/EB/NIBIB NIH HHS/United States
- NIH R01CA262630/National Institute of Health (NIH)
- National Institute for Health and Care Research
- National Institute of Health and Care Research (NIHR) University College London Hospital Biomedical Research Centre
- RP180404/Cancer Prevention and Research Institute of Texas
- P41 EB013598/EB/NIBIB NIH HHS/United States
- NIH/NCI Cancer Center Support Grant
- Center for Molecular Imaging and Bioengineering
- NIH P41EB013598/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous