The Effects of Ingesting a Single Bolus of Hydrolyzed Collagen versus Free Amino Acids on Muscle Connective Protein Synthesis Rates
- PMID: 40523226
- PMCID: PMC12520035
- DOI: 10.1249/MSS.0000000000003788
The Effects of Ingesting a Single Bolus of Hydrolyzed Collagen versus Free Amino Acids on Muscle Connective Protein Synthesis Rates
Abstract
Purpose: This study aimed to assess the effect of ingesting a single bolus of hydrolyzed collagen or free amino acids on myofibrillar and muscle connective protein synthesis rates.
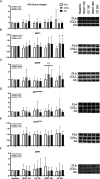
Methods: In a randomized, double-blind, parallel design, 45 young male ( n = 21) and female ( n = 24) adults (age, 23 ± 3 yr; BMI, 22.3 ± 2.2 kg·m -2 ) received intravenous infusions with L-[ ring - 13 C 6 ]-phenylalanine. After unilateral resistance exercise, participants ingested either 30 g hydrolyzed collagen (COLL, n = 15), 30 g free amino acids reflecting the collagen amino acid profile (AA, n = 15), or a noncaloric placebo (PLA, n = 15). Blood and muscle tissue samples were collected over 6 h to assess myofibrillar and muscle connective protein synthesis rates and associated signaling responses.
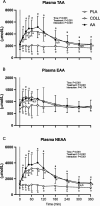
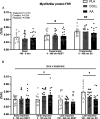
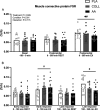
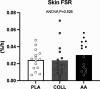
Results: Both collagen and free amino acid ingestion substantially increased circulating plasma amino acids concentrations and affected collagen turnover proteins. Collagen and free amino acid ingestion did not significantly increase myofibrillar protein synthesis rates in the rested (0.039 ± 0.011, 0.037 ± 0.010, and 0.036 ± 0.015%·h -1 in PLA, COLL, and AA, respectively) or the exercised (0.049 ± 0.010, 0.048 ± 0.011, and 0.045 ± 0.013%·h -1 ) leg ( P > 0.05). Similarly, both collagen and free amino acid ingestion did not significantly increase muscle connective protein synthesis rates in the rested (0.065 ± 0.014, 0.063 ± 0.017, and 0.061 ± 0.025%·h -1 in PLA, COLL, and AA, respectively) or the exercised (0.098 ± 0.023, 0.092 ± 0.028, and 0.085 ± 0.024%·h -1 ) leg ( P > 0.05).
Conclusions: The ingestion of a single bolus of collagen hydrolysate or free amino acids substantially increases circulating amino acids concentrations, particularly glycine, but does not further increase myofibrillar or muscle connective protein synthesis rates at rest or during recovery from exercise in healthy, recreationally active young men and women.
Keywords: CONNECTIVE TISSUE; GLYCINE; MYOFIBRILLAR PROTEINS; RESISTANCE EXERCISE.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American College of Sports Medicine.
Figures


References
-
- Koopman R, van Loon LJ. Aging, exercise, and muscle protein metabolism. J Appl Physiol (1985). 2009;106(6):2040–8. - PubMed
-
- Huijing PA. Muscle as a collagen fiber reinforced composite: a review of force transmission in muscle and whole limb. J Biomech. 1999;32(4):329–45. - PubMed
-
- Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol Endocrinol Metab. 1995;268(3):E514–20. - PubMed
-
- Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol Endocrinol Metab. 1997;273(1):E99–107. - PubMed

