Effects of Selective Head-and-Neck Cooling on Brain Injury-Related Biomarker Levels and Symptom Rating Following a Boxing Bout: Protocol for an Exploratory Randomized Trial
- PMID: 40523276
- PMCID: PMC12209727
- DOI: 10.2196/68954
Effects of Selective Head-and-Neck Cooling on Brain Injury-Related Biomarker Levels and Symptom Rating Following a Boxing Bout: Protocol for an Exploratory Randomized Trial
Abstract
Background: Head impacts are common in contact sports such as boxing and occur at times of elevated core body and brain temperatures induced by the exercise. Following impact, elevated brain temperature may lead to the development of exacerbated brain injury that can be monitored by blood biomarkers. Blood-brain biomarkers S100B and glial fibrillary acidic protein (GFAP) reflect glial injury; neurofilament light (NFL), axonal injury; and Neuron-Specific Enolase (NSE) and Tubulin-associated unit (tau), neuronal injury. Time to peak levels post injury for these biomarkers varies. Levels of S100B l peak early post injury, while NSE, GFAP, and tau are regarded as subacute markers, and NFL shows prolonged increases. We attempt to cover a large spectrum of first week postfight alterations in blood-brain biomarkers and their response to head-neck cooling.
Objective: We hypothesized that acute head-and-neck cooling, recently shown to shorten return-to-play in concussed ice hockey players, applied acutely following a boxing bout, is associated with an attenuated concentration of blood biomarkers and improved symptom rating.
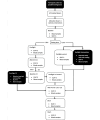
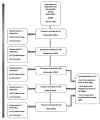
Methods: The trial is academically driven and funded by external and hospital research funds. Young, healthy elite boxers aged ≥18 years are recruited. Before, and immediately after a competitive boxing bout consisting of 2 or 3 rounds of 2 minutes each, blood samples are drawn. Boxers are randomized to intervention or control management by 1:1 allocation before baseline testing. After the initial postfight blood sample is drawn and symptom rating using the Sports Concussion Assessment Tool-5 (SCAT-5) has been collected, the boxers receive either acute selective head-and-neck cooling for 45 minutes or routine postfight management. The number of head impacts is counted in all boxers on match video recordings. In both groups, blood samples are drawn 45 minutes after the initial postbout blood sample, as well as 3 and 6 days post fight. At all blood sampling time points, the number of symptoms (NOS) and symptom severity score (SSS) are assessed using the symptom rating part of the SCAT-5. The primary endpoint is the difference in biomarker levels (GFAP, NFL, tau, UCH-L1, neuronal-specific enolase) immediately post fight and preintervention, to those obtained at 6 days post fight. The postfight SCAT-5 NOS and SSS are secondary endpoints.
Results: Recruitment started in November 2021 and is ongoing. So far, 41 boxers have been included: 20 controls and 21 cooled. Data collection started in October 2024 following the completion of blood sample analysis. We expect to recruit more boxers before the middle of 2025, but challenges with recruitment may limit this.
Conclusions: There is no treatment available for boxing-induced brain injury. Biomarkers are surrogate yet objective markers of brain injury, and the head-and-neck cooling treatment may attenuate the concentration of brain injury-related biomarkers as well as reduce symptoms induced by head impacts attained during a boxing fight.
Trial registration: ClinicalTrials.gov NCT06386484; https://clinicaltrials.gov/study/NCT06386484.
International registered report identifier (irrid): DERR1-10.2196/68954.
Keywords: biomarker; boxing; glial fibrillary acidic protein; neurofilament-light; sports concussion.
©Ali Al-Husseini, Yelverton Tegner, Kaj Blennow, Henrik Zetterberg, Niklas Marklund. Originally published in JMIR Research Protocols (https://www.researchprotocols.org), 16.06.2025.
Conflict of interest statement
Conflicts of Interest: YT is a scientific advisor for PolarCool Inc. Lund, Sweden. NM previously was a scientific advisor for PolarCool Inc. Lund, Sweden. Polar Cool provides the cooling helmet, although has not influenced the design of the study protocol. HZ has served at scientific advisory boards and as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Alzecure, Biogen, Cellectricon, Fujirebio, Lilly, Novo Nordisk, and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). KB has served as a consultant, on advisory boards, or data monitoring committees for Abcam, Axon, Biogen, and JOMDD/Shimadzu. Julius Clinical, Lilly, MagQu, Novartis, Prothena, Roche Diagnostics, and Siemens Healthineers, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. IR has served on advisory boards and given talks sponsored by Biogen, Bayer, Merck, Roche, GlaxoSmithKline, TEVA, Sanofi, and UCB.
Figures


Similar articles
-
Association of Blood-Based Biomarkers and 6-Month Patient-Reported Outcomes in Patients With Mild TBI: A CENTER-TBI Analysis.Neurology. 2025 Jan 14;104(1):e210040. doi: 10.1212/WNL.0000000000210040. Epub 2024 Dec 9. Neurology. 2025. PMID: 39652812 Free PMC article.
-
Potential Blood-based Biomarkers for Concussion.Sports Med Arthrosc Rev. 2016 Sep;24(3):108-15. doi: 10.1097/JSA.0000000000000117. Sports Med Arthrosc Rev. 2016. PMID: 27482776 Free PMC article.
-
Prognostic Value of Blood-Based Protein Biomarkers in Traumatic Brain Injury: A Living Systematic Review and Meta-Analysis.J Neurotrauma. 2025 Aug;42(15-16):1256-1286. doi: 10.1089/neu.2024.0620. Epub 2025 May 27. J Neurotrauma. 2025. PMID: 40432557 Review.
-
The impact of kidney function on Alzheimer's disease blood biomarkers: implications for predicting amyloid-β positivity.Alzheimers Res Ther. 2025 Feb 19;17(1):48. doi: 10.1186/s13195-025-01692-z. Alzheimers Res Ther. 2025. PMID: 39972340 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
References
-
- Patricios JS, Schneider KJ, Dvorak J, Ahmed OH, Blauwet C, Cantu RC, Davis GA, Echemendia RJ, Makdissi M, McNamee M, Broglio S, Emery CA, Feddermann-Demont N, Fuller GW, Giza CC, Guskiewicz KM, Hainline B, Iverson GL, Kutcher JS, Leddy JJ, Maddocks D, Manley G, McCrea M, Purcell LK, Putukian M, Sato H, Tuominen MP, Turner M, Yeates KO, Herring SA, Meeuwisse W. Consensus statement on concussion in sport: the 6th International Conference on Concussion in Sport-Amsterdam, October 2022. Br J Sports Med. 2023 Jun;57(11):695–711. doi: 10.1136/bjsports-2023-106898.57/11/695 - DOI - PubMed
-
- Goldfinger MH, Ling H, Tilley BS, Liu AKL, Davey K, Holton JL, Revesz T, Gentleman SM. The aftermath of boxing revisited: identifying chronic traumatic encephalopathy pathology in the original Corsellis boxer series. Acta Neuropathol. 2018 Dec;136(6):973–974. doi: 10.1007/s00401-018-1926-8. https://europepmc.org/abstract/MED/30377771 10.1007/s00401-018-1926-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

