Perspective: Implications of Docosahexaenoic Acid and Eicosapentaenoic Acid Supplementation on the Immune System during Cancer Chemotherapy: Perspectives from Current Clinical Evidence
- PMID: 40523478
- PMCID: PMC12276385
- DOI: 10.1016/j.advnut.2025.100464
Perspective: Implications of Docosahexaenoic Acid and Eicosapentaenoic Acid Supplementation on the Immune System during Cancer Chemotherapy: Perspectives from Current Clinical Evidence
Abstract
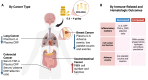
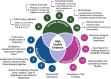
Docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) are omega-3 long-chain polyunsaturated fatty acids (n-3 LCPUFAs) with pleiotropic effects on the immune system. Although several preclinical studies support their potential to enhance cancer treatment efficacy, this has not yet been translated into clinical studies. Currently, there are no official recommendations for n-3 LCPUFAs supplementation during cancer chemotherapy. This review examined human studies that supplemented DHA and/or EPA in patients with cancer undergoing chemotherapy, aiming to evaluate n-3 LCPUFAs effects on immune outcomes. A systematic search was conducted using electronic databases, including OvidMedline and the Global Organization for EPA and DHA Omega-3s Clinical Study Database. Twelve studies were included in this review. EPA+DHA doses ranged from 0.6 to 4 g/d, and intervention durations ranged from 6 wk to 6 mo. Most of the studies demonstrated changes in some immune-related outcomes, including reductions in the blood markers of inflammation (interleukin-6 and C-reactive protein), a lower incidence of adverse events, and the preservation of immune cell concentrations and functions (phagocytosis and hydrogen peroxide production). However, caution is warranted due to the limited number of studies and the heterogeneity of study designs. This review discusses the limitations of current studies and the mechanistic evidence supporting the investigation and potential use of n-3 LCPUFAs during cancer chemotherapy. Future research should focus on addressing these limitations by conducting well-designed, large-scale clinical trials that clearly report the dose and duration of n-3 LCPUFAs supplementation during specific chemotherapy regimens. Despite some promising outcomes, more evidence will be needed before recommending n-3 LCPUFAs supplementation as part of chemotherapy regimens aimed at attenuating chemotherapy-induced immune alterations.
Keywords: chemotherapy; dietary supplements; docosahexaenoic acid; eicosapentaenoic acid; immune function; inflammation; omega-3 fatty acids.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest CJF is an Editorial Board Member of the Advances in Nutrition and played no role in the Journal’s evaluation of the manuscript. All other authors report no conflicts of interest.
Figures
References
-
- Bray F., Laversanne M., Sung H., Ferlay J., Siegel R.L., Soerjomataram I., et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024;74(3):229–263. - PubMed
-
- Society A.C. American Cancer Society (ACS); Atlanta: 2024. Cancer Facts & Figures 2024.
-
- Aboud K., Meissner M., Ocen J., Jones R. Cytotoxic chemotherapy: clinical aspects. Medicine. 2023;51(1):23–27.
-
- Livshits Z., Rao R.B., Smith S.W. An approach to chemotherapy-associated toxicity. Emerg. Med. Clin. North Am. 2014;32(1):167–203. - PubMed
-
- Dunn G.P., Bruce A.T., Ikeda H., Old L.J., Schreiber R.D. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 2002;3(11):991–998. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

