Targeted incorporation of collagen IV to the basement membrane: A step forward for developing extracellular protein therapies
- PMID: 40523614
- PMCID: PMC12274822
- DOI: 10.1016/j.jbc.2025.110384
Targeted incorporation of collagen IV to the basement membrane: A step forward for developing extracellular protein therapies
Abstract
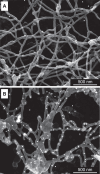
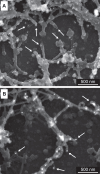
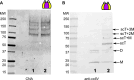
The collagen IV scaffold serves as a fundamental structural unit of the basement membrane (BM). Understanding its structure, assembly, and function is essential for tissue engineering, the design of organoid models, and developing therapies for diseases such as Alport syndrome, Gould syndrome, psoriasis, eye abnormalities, hearing loss, and others, where collagen IV is required for structural integrity and functionality of the BM. The collagen IV molecule is a 400 nm long heterotrimer, comprising non-collagenous 1 (NC1), collagenous, and 7S domains. The assembly of the collagen IV scaffold involves oligomerization of the C-terminal NC1 and the N-terminal 7S domains, along with lateral associations within the collagenous domain. However, the detailed architecture and assembly mechanisms of the collagen IV scaffold remain unclear. Here, we investigated the potency and mechanism of recombinant single-chain NC1 trimer incorporation into the collagen IV scaffold. We discovered that the NC1 trimer influences the overall assembly of the basement membrane by affecting the quality of the developing collagen IV scaffold in a dose-dependent manner, without impacting already established scaffolds. This interference occurs through the hexamerization of supplemented NC1 trimers with endogenous NC1 domains, as the NC1 trimer becomes sulfilimine crosslinked with the existing chains. Overall, the single-chain NC1 trimer of collagen IV is crucial for developing novel extracellular therapies in two main ways: (1) facilitating the delivery and incorporation of functional replacements like collagen IV fragments and (2) inhibiting the formation of new basement membranes in conditions such as tumor growth and detrimental vascularization.
Keywords: Alport syndrome; Gould syndrome; NC1 domain; basement membrane; collagen IV; molecular orthotics.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures






Similar articles
-
Collagen IV of basement membranes: III. Chloride pressure is a primordial innovation that drives and maintains the assembly of scaffolds.J Biol Chem. 2023 Nov;299(11):105318. doi: 10.1016/j.jbc.2023.105318. Epub 2023 Oct 4. J Biol Chem. 2023. PMID: 37797699 Free PMC article.
-
A chloride ring is an ancient evolutionary innovation mediating the assembly of the collagen IV scaffold of basement membranes.J Biol Chem. 2019 May 17;294(20):7968-7981. doi: 10.1074/jbc.RA119.007426. Epub 2019 Mar 28. J Biol Chem. 2019. PMID: 30923125 Free PMC article.
-
Type IV collagen derived non-collagenous domain α6 (IV) NC1 and its derivative fragments inhibit endothelial cell proliferation and attenuates in-vivo chorioallantoic membrane angiogenesis.Cytotechnology. 2025 Apr;77(2):47. doi: 10.1007/s10616-025-00709-7. Epub 2025 Jan 25. Cytotechnology. 2025. PMID: 39867830
-
Beyond classical collagen: basement membrane collagen IV in age-associated lung diseases.Eur Respir Rev. 2025 Jul 23;34(177):240192. doi: 10.1183/16000617.0192-2024. Print 2025 Jul. Eur Respir Rev. 2025. PMID: 40701642 Free PMC article. Review.
-
Genotype-Based Molecular Mechanisms in Alport Syndrome.J Am Soc Nephrol. 2025 Jun 1;36(6):1176-1183. doi: 10.1681/ASN.0000000647. Epub 2025 Feb 3. J Am Soc Nephrol. 2025. PMID: 39899372 Review.
References
-
- Parkin J.D., San Antonio J.D., Pedchenko V., Hudson B., Jensen S.T., Savige J. Mapping structural landmarks, ligand binding sites, and missense mutations to the collagen IV heterotrimers predicts major functional domains, novel interactions, and variation in phenotypes in inherited diseases affecting basement membranes. Hum. Mutat. 2011;32:127–143. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

