Cytochrome P450BM-3 and P450 11A1 retain Compound I (FeO3+) chemistry with electrophilic substrates poised for Compound 0 (Fe3+O2-) reactions
- PMID: 40523616
- PMCID: PMC12332404
- DOI: 10.1016/j.jbc.2025.110378
Cytochrome P450BM-3 and P450 11A1 retain Compound I (FeO3+) chemistry with electrophilic substrates poised for Compound 0 (Fe3+O2-) reactions
Abstract
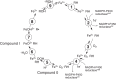
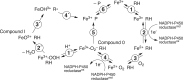
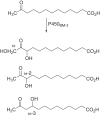
The catalytic cycle of cytochrome P450 (P450) enzymes involves ferric peroxide anion (Fe3+O2-, Compound 0) and perferryl oxygen (FeO3+, Compound I) intermediates. Compound I is generally viewed as responsible for most P450-catalyzed oxidations, but Compound 0 has been implicated in the oxidation of some carbonyl compounds, particularly deformylation reactions. We considered the hypothesis that Compound 0 could also attack other electrophilic carbon atoms and accordingly positioned keto groups at preferred hydroxylation sites of substrates for two P450s with well-defined catalytic reactions, bacterial P450BM-3 (102A1), and human P450 11A1. The predicted products of Compound I and Compound 0 reactions were analyzed. With the normally preferred ω-1 site blocked, P450BM-3 oxidized 12-oxotridecanoic acid (12-oxo C13:0) only at the ω-2 position (yielding 11-hydroxy,12-oxotridecanoic acid), indicative of a Compound I oxidation. P450 11A1 is highly selective for catalyzing the 22R-hydroxylation of cholesterol (and some other sterols) in the first step of its overall side-chain cleavage reaction. With 22-oxocholesterol as the substrate, P450 11A1 (slowly) generated only 23-hydroxy,22-oxocholesterol, indicative of Compound I oxidation. Neither P450 generated the products expected from nucleophilic Compound 0 reactions. We conclude that the strategic placement of electrophilic oxo substituents at sites of substrate hydroxylation failed to divert the oxidation mechanism to a Compound 0 pathway with either enzyme. Instead, the Compound I mechanism-blocked at the preferred reaction site-was redirected to neighboring carbons, suggesting that the basis for Compound 0-mediated reactions lies in chemical properties of the enzyme rather than those of the substrate.
Keywords: 11A1; BM-3; CYP; Compound 0; Compound I; cytochrome P450; enzyme mechanism; fatty acid oxidation; steroid oxidation.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures







Similar articles
-
Unique structural features define the decarboxylation activity of a CYP152 fatty acid decarboxylase from Lacicoccus alkaliphilus.J Biol Chem. 2025 Jul;301(7):110397. doi: 10.1016/j.jbc.2025.110397. Epub 2025 Jun 19. J Biol Chem. 2025. PMID: 40543591 Free PMC article.
-
Liver fatty acid binding protein FABP1 transfers substrates to cytochrome P450 4A11 for catalysis.J Biol Chem. 2025 Feb;301(2):108168. doi: 10.1016/j.jbc.2025.108168. Epub 2025 Jan 8. J Biol Chem. 2025. PMID: 39793892 Free PMC article.
-
A flexible linker of 8-amino acids between the membrane binding segment and the FMN domain of cytochrome P450 reductase is necessary for optimal activity.J Inorg Biochem. 2024 Oct;259:112667. doi: 10.1016/j.jinorgbio.2024.112667. Epub 2024 Jul 16. J Inorg Biochem. 2024. PMID: 39032346
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article.
References
-
- Ortiz de Montellano P.R., editor. Cytochrome P450: Structure, Mechanism, and Biochemistry. 4th Ed. Springer; New York: 2015.
-
- Guengerich F.P. Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity. Chem. Res. Toxicol. 2001;14:611–650. - PubMed
-
- Ortiz de Montellano P.R. In: Cytochrome P450: Structure, Mechanism, and Biochemistry. 4th Ed. Ortiz de Montellano P.R., editor. Springer; New York: 2015. Substrate oxidation; pp. 111–176.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

