Efficacy of subanaesthetic esketamine on the prevention of postoperative delirium in older adult patients after cardiovascular surgery: protocol for a single-centre, randomised, double-blind, placebo-controlled trial (SEPDOC trial) in China
- PMID: 40523785
- PMCID: PMC12314811
- DOI: 10.1136/bmjopen-2024-089719
Efficacy of subanaesthetic esketamine on the prevention of postoperative delirium in older adult patients after cardiovascular surgery: protocol for a single-centre, randomised, double-blind, placebo-controlled trial (SEPDOC trial) in China
Abstract
Introduction: Postoperative delirium (POD) is a common and serious complication in older adult patients undergoing cardiovascular surgery. Esketamine is known for its anti-inflammatory and neuroprotective properties. While it has shown preventive effects on POD in those not undergoing cardiovascular surgery, its efficacy in older adult patients undergoing cardiovascular surgery remains uncertain. Therefore, we herein aimed to evaluate the preventive effect of intraoperative subanaesthetic esketamine on POD in this specific population.
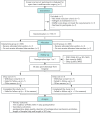
Methods and analysis: This single-centre, randomised, double-blind, placebo-controlled trial will enrol 778 patients aged 60-80 years undergoing open-heart cardiovascular surgery in China, from September 2023 to December 2025. The participants will be randomly assigned in a 1:1 ratio to the following groups: the esketamine group and the control group. In the esketamine group, esketamine (2 mg/mL) will be administered intravenously at a dosage of 0.3 mg/kg over 10 min following tracheal intubation, followed by a continuous infusion at 0.15 mg/kg/h until the end of the surgery. Patients in the control group will receive a placebo following the same dosage and regimen. The incidence of POD will be the primary outcome and will be assessed twice daily from the first to the seventh postoperative day. The postoperative sleep quality, duration of postoperative mechanical ventilation, and length of hospital and intensive care unit stay will be the secondary outcomes.
Ethics and dissemination: Ethical approval was obtained from the Institutional Review Board of Fuwai Central China Cardiovascular Hospital (No. 2023068). Public disclosure is guaranteed post-trial, and the results will be published in a peer-reviewed scientific journal.
Trial registration number: ChiCTR2300074395.
Keywords: Aged; Anaesthesia in cardiology; Cardiovascular Disease; Clinical Protocols; Delirium.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
The effects of esketamine on postoperative delirium in older patients with fragile brain function during the non-acute phase following lung cancer surgery: a randomized controlled trial.BMC Geriatr. 2025 Aug 9;25(1):608. doi: 10.1186/s12877-025-06268-y. BMC Geriatr. 2025. PMID: 40783518 Free PMC article. Clinical Trial.
-
Effect of opioid-free anaesthesia on postoperative delirium in elderly patients undergoing gastrointestinal surgery: study protocol for a single-centre, prospective, randomized controlled trial.BMC Geriatr. 2025 Jul 28;25(1):554. doi: 10.1186/s12877-025-06145-8. BMC Geriatr. 2025. PMID: 40721742 Free PMC article.
-
Combination of dexmedetomidine and esketamine for postoperative nausea and vomiting in patients undergoing laparoscopic surgery: study protocol for a prospective, randomized, controlled trial.Trials. 2025 Jul 1;26(1):230. doi: 10.1186/s13063-025-08935-2. Trials. 2025. PMID: 40598630 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Processed electroencephalogram and evoked potential techniques for amelioration of postoperative delirium and cognitive dysfunction following non-cardiac and non-neurosurgical procedures in adults.Cochrane Database Syst Rev. 2018 May 15;5(5):CD011283. doi: 10.1002/14651858.CD011283.pub2. Cochrane Database Syst Rev. 2018. PMID: 29761891 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical