Transcription arrest induces formation of RNA granules in mitochondria
- PMID: 40523799
- PMCID: PMC12171109
- DOI: 10.26508/lsa.202403082
Transcription arrest induces formation of RNA granules in mitochondria
Abstract
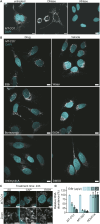
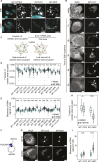
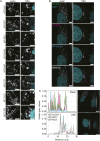
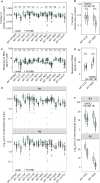
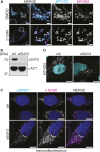
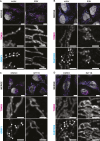
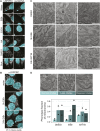
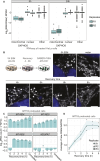
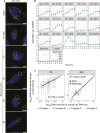
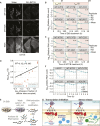
Mitochondrial gene expression regulation is required for the biogenesis of oxidative phosphorylation (OXPHOS) complexes, yet the spatial organization of mitochondrial RNAs (mt-RNAs) remains unknown. Here, we investigated the spatial distribution of mt-RNAs during various cellular stresses using single-molecule RNA-FISH. We discovered that transcription inhibition leads to the formation of distinct RNA granules within mitochondria, which we term inhibition granules. These structures differ from canonical mitochondrial RNA granules and form in response to multiple transcription arrest conditions, including ethidium bromide treatment, specific inhibition or stalling of the mitochondrial RNA polymerase, and depletion of the SUV3 helicase. Inhibition granules appear to stabilize certain mt-mRNAs during prolonged transcription inhibition. This phenomenon coincides with an imbalance in OXPHOS complex expression, where mitochondrial-encoded transcripts decrease while nuclear-encoded subunits remain stable. We found that cells recover from transcription inhibition via resolving the granules, restarting transcription, and repopulating the mitochondrial network with mt-mRNAs within hours. We suggest that inhibition granules may act as a reservoir to help overcome OXPHOS imbalance during recovery from transcription arrest.
© 2025 Hansen et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Clu1/Clu form mitochondria-associated granules upon metabolic transitions and regulate mitochondrial protein translation via ribosome interactions.PLoS Genet. 2025 Jul 7;21(7):e1011773. doi: 10.1371/journal.pgen.1011773. eCollection 2025 Jul. PLoS Genet. 2025. PMID: 40623095 Free PMC article.
-
Post-transcriptional modifications and regulation of mRNAs in human mitochondria.Biochimie. 2025 Jun 25:S0300-9084(25)00128-2. doi: 10.1016/j.biochi.2025.06.015. Online ahead of print. Biochimie. 2025. PMID: 40578749 Review.
-
Role of the SAF-A/HNRNPU SAP domain in X chromosome inactivation, nuclear dynamics, transcription, splicing, and cell proliferation.PLoS Genet. 2025 Jun 10;21(6):e1011719. doi: 10.1371/journal.pgen.1011719. eCollection 2025 Jun. PLoS Genet. 2025. PMID: 40493679 Free PMC article.
-
Super-resolution microscopy of mitochondrial mRNAs.Nat Commun. 2025 Jul 10;16(1):6391. doi: 10.1038/s41467-025-61577-5. Nat Commun. 2025. PMID: 40640125 Free PMC article.
-
Assessing the comparative effects of interventions in COPD: a tutorial on network meta-analysis for clinicians.Respir Res. 2024 Dec 21;25(1):438. doi: 10.1186/s12931-024-03056-x. Respir Res. 2024. PMID: 39709425 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
