Neuroprotective Effects of a Benzofuran-Containing Selenium in a Mouse Alzheimer's Disease Model: Molecular, Biochemical, and Behavioral Analyses
- PMID: 40523837
- PMCID: PMC12232306
- DOI: 10.1021/acschemneuro.5c00139
Neuroprotective Effects of a Benzofuran-Containing Selenium in a Mouse Alzheimer's Disease Model: Molecular, Biochemical, and Behavioral Analyses
Abstract
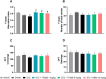
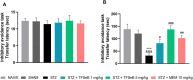
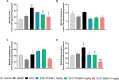
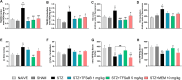
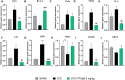
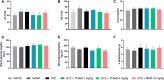
Alzheimer's disease (AD) is a neurodegenerative disorder mainly characterized by progressive cognitive decline, for which effective treatments remain limited, and selenium is known for its neuroprotective actions. Thus, this study evaluated the neuroprotective effects of the compound 2-(((3-trifluoromethyl)phenyl(selenyl)methyl)-2,3-dihydrobenzofuran (TFSeB) in a streptozotocin (STZ)-induced AD model in male Swiss mice. The animals received intracerebroventricular injections of STZ (3 mg/kg, a neurotoxic agent) to induce cognitive deficits, followed by treatment with TFSeB (1 and 5 mg/kg, intragastrically). Behavioral tests revealed that, like positive control (memantine), the compound TFSeB improved memory performance in the Y-maze, novel object recognition, and passive avoidance tests, suggesting its ability to counteract STZ-induced memory impairments. Biochemical analyses showed that the compound reduced oxidative stress markers in the prefrontal cortex and cerebellum of mice exposed to STZ, including TBARS, ROS, and nitrite levels while increasing NPSH. STZ induced an increase in monoamine oxidase B (MAO-B) activity in the hippocampus and cortex, as well as in acetylcholinesterase (AChE) activity in the cortex and cerebellum, which were reverted by TFSeB. Hippocampal RT-qPCR molecular analyses revealed that TFSeB modulated apoptosis-related proteins by increasing BCL-2 and decreasing BAX expression, favoring neuronal survival. Moreover, TFSeB increased brain-derived neurotrophic factor (BDNF) and nuclear factor erythroid 2 (NRF2), targets associated with neuroprotection. The compound also decreased key inflammatory and neurodegenerative markers, including nuclear factor kappa B (NF-κB), interleukin-6 (IL-6), and glycogen synthase kinase 3 beta (GSK3B). In conclusion, the compound TFSeB demonstrates promising protective effects in a STZ-induced AD model by modulating key neurochemical, oxidative, and neuroinflammatory pathways.
Keywords: memory; neuroinflammation; oxidative damage; selenium; streptozotocin.
Figures

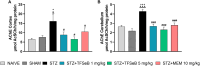


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

