Garsorasib, a KRAS G12C inhibitor, with or without cetuximab, an EGFR antibody, in colorectal cancer cohorts of a phase II trial in advanced solid tumors with KRAS G12C mutation
- PMID: 40523897
- PMCID: PMC12170901
- DOI: 10.1038/s41392-025-02274-z
Garsorasib, a KRAS G12C inhibitor, with or without cetuximab, an EGFR antibody, in colorectal cancer cohorts of a phase II trial in advanced solid tumors with KRAS G12C mutation
Abstract
Mutations in the KRAS gene have long been implicated in the pathogenesis of colorectal cancer (CRC). KRAS G12C inhibitors overcome the "undruggable" challenge, enabling precision therapy. Garsorasib (D-1553), a highly potent and selective KRAS G12C inhibitor, has demonstrated promising anti-tumor activity and favorable safety profile in early clinical trials. We conducted an open-label, nonrandomized phase II trial (ClinicalTrials.gov, NCT04585035) to assess the safety and efficacy of garsorasib with or without cetuximab in KRAS G12C-mutated CRC. In the monotherapy cohort (n = 26), objective response rate (ORR) was 19.2% (95% CI, 6.6-39.4), disease control rate (DCR) was 92.3% (95% CI, 74.9-99.1), median progression-free survival (PFS) was 5.5 months (95% CI, 2.9-11.6) and median overall survival (OS) was 13.1 months (95% CI, 9.5-NE). In the combination cohort (n = 42), ORR was 45.2% (95% CI, 29.8-61.3), DCR was 92.9% (95% CI, 80.5-98.5), median PFS was 7.5 months (95% CI, 5.5-8.1), and median OS was not reached. Grade ≥3 treatment-related adverse events occurred in 5 (19.2%) and 6 (14.3%) patients in monotherapy and combination cohort, respectively. Garsorasib with or without cetuximab showed a promising efficacy and manageable safety profiles in heavily pretreated patients with KRAS G12C-mutated CRC, providing a potential new treatment approach for such population.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: K-W.L. disclosed institutional research funding (to the institution) from InventisBio for conducting the clinical trial related to this report; research funding (to the institution; outside of the submitted work) for conducting clinical trials from AstraZeneca, Merck Sharp and Dohme, ONO pharmaceutical, Merck KGaA, Roche, BeiGene, Leap therapeutics, ALX Oncology, Zymeworks, Astellas, Macrogenics, Amgen, Seagen, Bolt therapeutics, Trishula therapeutics, MedPacto, Green Cross Corp, Y-BIOLOGICS, Daiichi Sankyo, Taiho Pharmaceutical, Elevar Therapeutics, Metafines, Idience, Genome & Company, Exelixis, Panolos Bioscience; has participated on data safety monitoring boards or advisory boards for ALX Oncology and Metafines; has received consulting fees from Daiichi Sankyo, Merck Sharp and Dohme, Astellas, Bayer (outside the submitted work); and honoraria for lectures or presentation from Merck KGaA, Daiichi Sankyo, Astellas, Sanofi-Aventis (outside the submitted work). M.M. reports study payments from InventisBio; reports consulting fees from Roche, Bristol-Myers Squibb, AstraZeneca, Pfizer, Merck Serono, Guardant Health, The Limbic, Beigene, Amgen, Merck, IQVIA, and Eli Lilly, participates on a Data Safety Monitoring Board or Advisory Board for Novartis. S.M.G. reports consulting fees from Pfizer, Takeda, Boehringer-Ingelheim, Astra-Zeneca, Genentech/Roche, Daichii, Abbvie, Arcus, Blueprint, Mirati, Merck, Esai, Lilly, Novartis, Bayer, Gilead; reports support for attending meetings and/or travel from Merck, Mirati; participates on advisory board for Astra-Zeneca. R.E.S. reports grants or contracts from AstraZeneca and Merck; reports consulting fees from GlaxoSmithKline, AstraZeneca, Janssen Oncology, Macrogenics, Daiichi, Sanofi, BeiGene, Gilead, Regeneron, Targeted Oncology, G1 Therapeutics, GE HealthCare, Amgen, and Lilly Oncology; reports Payment or honoraria for lectures, presentations from EMD Serono, Illumina, GameOn!, OncLive, Binay Foundation, APP Oncology, and Masters in Thoracic Oncology Summit; participates on advisory board for GlaxoSmithKline, AstraZeneca, Janssen Oncology, Macrogenics, Daiichi, Sanofi, BeiGene, Gilead, Regeneron, Targeted Oncology, G1 Therapeutics, GE HealthCare, Amgen, and Lilly Oncology. Y.Z., L.L. and X.X. report InventisBio employment. Z.X., Z.S., Y.W. and L.Z. report InventisBio employment and stock. R.-H.X. reports speaker fees from Bristol Myers Squibb, Roche, MerckSerono, Hutchison, Hengrui, Junshi, Qilu, CPPC, Henlius, and participates on advisory board for Astellas, MSD, AstraZeneca, Junshi, Hengrui, BeiGene. Innovent, CPPC, and Keymed. All other authors declare no competing interests.
Figures
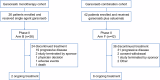
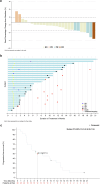
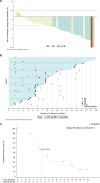
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

