High tumor expression of CTLA4 identifies lymph node-negative basal-like breast cancer patients with excellent prognosis
- PMID: 40523912
- PMCID: PMC12170890
- DOI: 10.1038/s43856-025-00865-z
High tumor expression of CTLA4 identifies lymph node-negative basal-like breast cancer patients with excellent prognosis
Abstract
Background: Tumor immune cell infiltration is a favorable prognostic factor in triple-negative breast cancer. Most triple-negative tumors belong to the aggressive basal-like subtype. We hypothesized that immune gene expression may identify low-risk patients for whom adjuvant chemotherapy can be de-escalated.
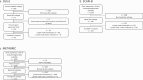
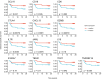
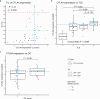
Methods: The expression of 753 immune-related genes was analyzed in tumor biopsies from 45 patients with basal-like disease and no lymph node metastases (Oslo1 cohort) and evaluated for prognostic value. Findings were validated in two independent cohorts. Oslo1 biopsies were also analyzed for tumor-infiltrating lymphocytes (TIL) and tertiary lymphoid structures (TLS).
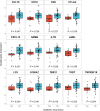
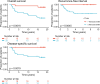
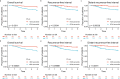
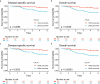
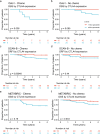
Results: Here we show that a high expression of CTLA4 (above 63rd percentile) is associated with an excellent prognosis in the Oslo1 cohort. None of the patients in the CTLA4high group suffered disease recurrence (median follow-up 7.4 years) or breast cancer-related death (median follow-up 17.7 years). Analysis of the SCAN-B (n = 233; 97% without distant recurrence in CTLA4high group) and METABRIC cohorts (n = 155; 93% disease-specific survival in CTLA4high group) validates this finding, which also applies to patients who did not receive chemotherapy. CTLA4 expression correlates with TIL score and TLS levels (Oslo1 cohort), but no TILlow/CTLA4high patients died from breast cancer, suggesting that the CTLA4 readout identifies low-risk patients not captured by TIL assessment.
Conclusions: A high primary tumor expression of CTLA4 identifies patients with an excellent prognosis, for whom standard chemotherapy may be de-escalated or omitted.
Plain language summary
Most breast cancer patients are cured after the initial surgery and radiotherapy. However, basal-like breast cancer, which makes up about 15% of cases, carries a high risk of early recurrence and death. Nearly all patients with basal-like breast cancer therefore receive additional treatment before and after surgery, including chemotherapy and immunotherapy. This treatment reduces the risk of recurrence but causes considerable short- and long-term side effects. In the current study, we found that patients with high levels of the immune-related gene CTLA4 in tumor had an excellent prognosis, likely because the immune system aids in fighting the cancer. Our findings suggest that the additional treatments given to some patients may safely be omitted or reduced, which could improve the quality of life of cancer survivors.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: A.H.R., J.A.K., X.T., V.K., B.N., O.C.L. and H.G.R. are inventors on a patent application related to the work described in this article. J.A.K. has received research support from NanoString, Bristol Myers Squibb, F. Hoffmann-La Roche, and NEC OncoImmunity. The other authors declare no competing interests.
Figures
References
-
- Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.71, 209–249 (2021). - PubMed
-
- Deluche, E. et al. Contemporary outcomes of metastatic breast cancer among 22,000 women from the multicentre ESME cohort 2008-2016. Eur. J. Cancer129, 60–70 (2020). - PubMed
-
- Emens, L. A. et al. First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: IMpassion130 final overall survival analysis. Ann. Oncol.32, 983–993 (2021). - PubMed
-
- Cortes, J. et al. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N. Engl. J. Med.387, 217–226 (2022). - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

