Phosphorylation of nucleosides by P-N bond species generated from prebiotic reduced phosphorus sources
- PMID: 40523969
- PMCID: PMC12170892
- DOI: 10.1038/s42004-025-01577-0
Phosphorylation of nucleosides by P-N bond species generated from prebiotic reduced phosphorus sources
Abstract
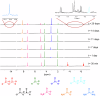
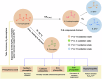
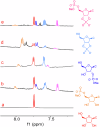
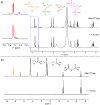
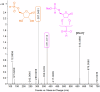
P-N species e.g., amidophosphates readily phosphorylate organics, thereby overcoming the so-called 'Phosphate Problem'. However, the formation of amidophosphates by plausible early Earth geochemical pathways is limited. We herein show that ammonolysis of the prebiotically plausible dimer of phosphite, pyrophosphite, readily affords amidophosphite, the monomeric P-N derivative of phosphite. Amidophosphite then undergoes spontaneous oxidation to form monoamidophosphate (MAP) and diamidophosphate (DAP) at room temperature (yields of the inorganic P-N species up to 48%). Oxidation of amidophosphite is promoted by O2, H2O2, ClO⁻ and by UV light irradiation (365 nm). Both amidophosphite and MAP and the crude reaction mixture react with nucleosides to form nucleotides with both phosphate and H-phosphonate (yields up to 65%) at 80 °C in the presence of urea, showing that monoamidated phosphorus compounds also willingly promote prebiotic reactions. This observation expands the range of P-N phosphorylating agents that can play a role in the chemical evolution of prebiotic molecules on the early Earth.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Gulick, A. Phosphorus as a factor in the origin of life. Am. Sci.43, 479–489 (1955).
-
- Pasek, M. A. A role for phosphorus redox in emerging and modern biochemistry. Curr. Opin. Chem. Biol.49, 53–58 (2019). - PubMed
-
- Gull, M. Prebiotic phosphorylation reactions on the early Earth. Challenges5, 193–212 (2014).
-
- Krishnamurthy, R., Guntha, S. & Eschenmoser, A. Regioselective alpha phosphorylation of aldoses in aqueous solution. Angew. Chem. Int. Ed.39, 2281–2285 (2000). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

