Thyrotropin exacerbates insulin resistance by triggering macrophage inflammation in subclinical hypothyroidism
- PMID: 40523992
- PMCID: PMC12229657
- DOI: 10.1038/s12276-025-01478-1
Thyrotropin exacerbates insulin resistance by triggering macrophage inflammation in subclinical hypothyroidism
Abstract
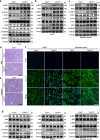
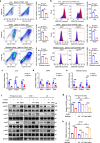
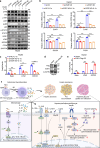
In subclinical hypothyroidism, the levels of serum thyroid-stimulating hormone (TSH) are positively correlated with insulin resistance; however, the precise mechanism is unclear. Except for thyroid follicular epithelial cells, macrophages express the highest levels of TSHR. Thus, we speculate that TSH may promote insulin resistance by triggering macrophage inflammation. Here we established a mouse model of TSH receptor (Tshr) myeloid-specific knockout (TshrMKO) and found that TshrMKO mice showed improvement on high-fat diet-induced obesity and insulin resistance compared with wild-type mice (Tshrf/f). In addition, TshrMKO mice exhibited decreased infiltration and M1 polarization of macrophages in liver, adipose and skeletal muscle. Co-culture experiments proved that Tshr-deficient macrophages decreased gluconeogenesis in hepatocytes but increased glucose uptake in adipocytes and skeletal muscle cells by improving the insulin signaling pathway. Mechanistically, increased TSH levels in subclinical hypothyroidism promoted the secretion of cytokines IL-1α, IL-1β and IL-6 by inducing macrophage M1 polarization, which upregulated EGR1 to transcriptionally activate LCN2 and SOCS3 in insulin target cells, thereby exacerbating insulin resistance. These effects could be reversed by IL-1 and IL-6 blockers IL-1RA and IL-6ST. Thus, we provided mechanistic insights into the predisposition to insulin resistance in subclinical hypothyroidism and revealed the role of TSH in metabolic disorders.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Study approval: Protocols for animal research were approved by the Institutional Animal Ethics Committee of Xi’an Jiaotong University. In compliance with the Declaration of Helsinki, patients were anonymously coded for human experimentation involving specimens. The Institutional Review Board and Human Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University authorized the study.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

