The dawn of a new era: can machine learning and large language models reshape QSP modeling?
- PMID: 40524056
- PMCID: PMC12170689
- DOI: 10.1007/s10928-025-09984-5
The dawn of a new era: can machine learning and large language models reshape QSP modeling?
Abstract
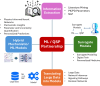
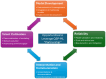
Quantitative Systems Pharmacology (QSP) has emerged as a cornerstone of modern drug development, providing a robust framework to integrate data from preclinical and clinical studies, enhance decision-making, and optimize therapeutic strategies. By modeling biological systems and drug interactions, QSP enables predictions of outcomes, optimization of dosing regimens, and personalized medicine applications. Recent advancements in artificial intelligence (AI) and machine learning (ML) hold the potential to significantly transform QSP by enabling enhanced data extraction, fostering the development of hybrid mechanistic ML models, and supporting the introduction of surrogate models and digital twins. This manuscript explores the transformative role of AI and ML in reshaping QSP modeling workflows. AI/ML tools now enable automated literature mining, the generation of dynamic models from data, and the creation of hybrid frameworks that blend mechanistic insights with data-driven approaches. Large Language Models (LLMs) further revolutionize the field by transitioning AI/ML from merely a tool to becoming an active partner in QSP modeling. By facilitating interdisciplinary collaboration, lowering barriers to entry, and democratizing QSP workflows, LLMs empower researchers without deep coding expertise to engage in complex modeling tasks. Additionally, the integration of Artificial General Intelligence (AGI) holds the potential to autonomously propose, refine, and validate models, further accelerating innovation across multiscale biological processes. Key challenges remain in integrating AI/ML into QSP workflows, particularly in ensuring rigorous validation pipelines, addressing ethical considerations, and establishing robust regulatory frameworks to address the reliability and reproducibility of AI-assisted models. Moreover, the complexity of multiscale biological integration, effective data management, and fostering interdisciplinary collaboration present ongoing hurdles. Despite these challenges, the potential of AI/ML to enhance hybrid model development, improve model interpretability, and democratize QSP modeling offers an exciting opportunity to revolutionize drug development and therapeutic innovation. This work highlights a pathway toward a transformative era for QSP, leveraging advancements in AI and ML to address these challenges and drive innovation in the field.
Keywords: Artificial Intelligence (AI); Drug Development; Hybrid Modeling; Large Language Models (LLMs); Machine Learning (ML); Model-Informed Drug Development (MIDD); Quantitative Systems Pharmacology (QSP).
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Leveraging large language models to compare perspectives on integrating QSP and AI/ML.J Pharmacokinet Pharmacodyn. 2025 May 5;52(3):29. doi: 10.1007/s10928-025-09976-5. J Pharmacokinet Pharmacodyn. 2025. PMID: 40325298
-
AML diagnostics in the 21st century: Use of AI.Semin Hematol. 2025 Jun 16:S0037-1963(25)00027-7. doi: 10.1053/j.seminhematol.2025.06.002. Online ahead of print. Semin Hematol. 2025. PMID: 40617702
-
Stench of Errors or the Shine of Potential: The Challenge of (Ir)Responsible Use of ChatGPT in Speech-Language Pathology.Int J Lang Commun Disord. 2025 Jul-Aug;60(4):e70088. doi: 10.1111/1460-6984.70088. Int J Lang Commun Disord. 2025. PMID: 40627744 Review.
-
Revolutionizing drug discovery: Integrating artificial intelligence with quantitative systems pharmacology.Drug Discov Today. 2025 Aug 6:104448. doi: 10.1016/j.drudis.2025.104448. Online ahead of print. Drug Discov Today. 2025. PMID: 40774584 Review.
-
Advancing personalized healthcare: leveraging explainable AI for BPPV risk assessment.Health Inf Sci Syst. 2024 Nov 24;13(1):1. doi: 10.1007/s13755-024-00317-3. eCollection 2025 Dec. Health Inf Sci Syst. 2024. PMID: 39606094
References
-
- Allerheiligen S, Abernethy D, Altman RB, Brouwer K, Califano A, David Z, D’argenio, Iyengar R, Jusko W, Lalonde R, Lauffenburger D, Shoichet B, Stevens J, Sorger P, Subramaniam S, Graaf PD, Vicini P, Ward RJ (2011) Quantitative and Systems Pharmacology in the Post-genomic Era: New Approaches to Discovering Drugs and Understanding Therapeutic. In: An NIH White Paper by the QSP Workshop Group
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

