The crucial role of metabolic reprogramming in driving macrophage conversion in kidney disease
- PMID: 40524149
- PMCID: PMC12172235
- DOI: 10.1186/s11658-025-00746-2
The crucial role of metabolic reprogramming in driving macrophage conversion in kidney disease
Abstract
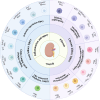
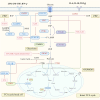
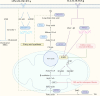
Interstitial fibrosis after acute kidney injury is an ongoing pathological process of chronic inflammatory injury and repair. Macrophages participate in renal inflammation, repair and fibrosis by continuously changing their phenotype and function. The tissue microenvironment of kidney injury induces changes in key metabolic enzymes, pathways and metabolites in macrophages, leading to phenotypic and functional conversions, but the detailed mechanisms are unclear. However, in the early phase of acute kidney injury, macrophages shift to a pro-inflammatory role relying on glycolysis and pentose phosphate pathways. The tissue microenvironment regulates the suppression of glycolysis-related genes and the up-regulation of oxidative phosphorylation and tricarboxylic acid cycle genes in macrophages, resulting in a gradual shift to an anti-inflammatory phenotype, which is involved in tissue repair and remodelling. In the late stage of injury, if macrophages continue to be overactive, they will be involved in renal fibrosis. The concomitant enhancement of nucleotide and amino acid metabolism, especially arginine and glutamine metabolism, is critical for the macrophage function and phenotypic transition during the above injury process. Macrophage metabolic reprogramming therefore provides new therapeutic targets for intervention in inflammatory injury and interstitial fibrosis in kidney disease.
Keywords: Inflammation; Injury and repair; Kidney fibrosis; Macrophages; Metabolic reprogramming.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. This manuscript does not contain any studies with human participants or animals performed by any of the authors. Consent for publication: Not applicable. This manuscript does not include details, images, or videos relating to an individual person. Competing interests: All the authors declared no competing interests.
Figures
Similar articles
-
Advances in macrophage metabolic reprogramming in myocardial ischemia-reperfusion.Cell Signal. 2024 Nov;123:111370. doi: 10.1016/j.cellsig.2024.111370. Epub 2024 Aug 30. Cell Signal. 2024. PMID: 39216681 Review.
-
Enhancement of angiotensin II type 1 receptor-associated protein suppresses kidney inflammation in a mouse model of aristolochic acid nephropathy.Sci Rep. 2025 Jul 31;15(1):27975. doi: 10.1038/s41598-025-08642-7. Sci Rep. 2025. PMID: 40744957 Free PMC article.
-
Postischemic inactivation of HIF prolyl hydroxylases in endothelium promotes maladaptive kidney repair by inducing glycolysis.J Clin Invest. 2024 Dec 2;135(3):e176207. doi: 10.1172/JCI176207. J Clin Invest. 2024. PMID: 39621585 Free PMC article.
-
Alveolar and Bone Marrow-derived Macrophages Differ in Metabolism and Glutamine Utilization.Am J Respir Cell Mol Biol. 2025 May;72(5):563-577. doi: 10.1165/rcmb.2023-0249OC. Am J Respir Cell Mol Biol. 2025. PMID: 39499818
-
Mechanisms of Acute Kidney Injury-Chronic Kidney Disease Transition: Unraveling Maladaptive Repair and Therapeutic Opportunities.Biomolecules. 2025 May 29;15(6):794. doi: 10.3390/biom15060794. Biomolecules. 2025. PMID: 40563434 Free PMC article. Review.
References
-
- Lovisa S, Zeisberg M, Kalluri R. Partial epithelial-to-mesenchymal transition and other new mechanisms of kidney fibrosis. Trends Endocrinol Metab. 2016;27(10):681–95. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

