4-Octyl itaconate alleviates endothelial cell inflammation and barrier dysfunction in LPS-induced sepsis via modulating TLR4/MAPK/NF-κB signaling : 4-Octyl itaconate alleviates endothelial dysfunction
- PMID: 40524158
- PMCID: PMC12168283
- DOI: 10.1186/s10020-025-01160-2
4-Octyl itaconate alleviates endothelial cell inflammation and barrier dysfunction in LPS-induced sepsis via modulating TLR4/MAPK/NF-κB signaling : 4-Octyl itaconate alleviates endothelial dysfunction
Abstract
Aim: Sepsis-induced vascular injury is a major contributor to the high mortality rate of sepsis. However, effective treatments remain elusive due to limited knowledge regarding the underlying molecular mechanisms. Itaconic acid, an endogenous metabolite, involved in multiple inflammatory diseases, but its role in sepsis-induced vascular injury remains unclear. The current study investigates the effect of 4-octyl itaconate (4-OI), a cell-permeable derivative of itaconic acid, on sepsis-induced vascular injury and organ damage.
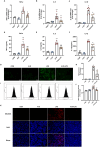
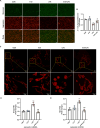
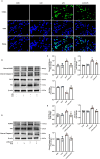
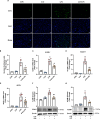
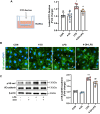
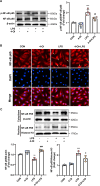
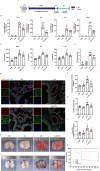
Methods and results: An in vitro cell model was established by treating human umbilical vein endothelial cells (HUVECs) with lipopolysaccharide (LPS). Quantitative reverse transcription polymerase chain reaction (qRT-PCR) and enzyme-linked immunosorbent assay (ELISA) revealed that 4-OI inhibited the LPS-induced increases in TNF-α, IL-6, and IL-1β levels. Cellular reactive oxygen species (ROS) levels, measured using the fluorescent probe DCFH-DA, mitochondrial ROS (mtROS) levels, measured by MitoSOX, and mitochondrial membrane potential (ΔΨ), detected by the fluorescent indicator JC-1, were all reduced following 4-OI treatment. Additionally, mtDNA release, detected by qRT-PCR, were decreased. Mitochondrial morphology, assessed by PK Mito Orange, was preserved by 4-OI treatment. Furthermore, 4-OI suppressed HUVECs apoptosis and pyroptosis, as detected by TUNEL staining and western blotting. 4-OI treatment also significantly inhibited LPS-induced cell adhesion, as shown in THP-1 attachment assay, by decreasing ICAM-1 and VCAM-1 expression. Cell permeability, determined by FITC-Dx-70 leakage, revealed that 4-OI effectively suppressed LPS-induced increases in cell permeability. Furthermore, 4-OI inhibited LPS-induced phosphorylation and internalization of VE-cadherin protein, preserving the adhesion junctions between endothelial cells. Network pharmacology and molecular docking analysis suggested the involvement of TLR4/MAPK/NF-κB signaling pathway as a key mechanism by which 4-OI ameliorated sepsis-induced vascular cell inflammation and injury, which was confirmed by western blotting. The in vitro results were subsequently verified in vivo in an LPS-induced sepsis mouse model. 4-OI pretreatment substantially decreased inflammatory cytokine levels in serum and lung tissues, inhibited pulmonary oedema and pulmonary vascular leakage, as evidenced by the wet-to-dry weight ratio and Evans blue staining of lung tissues, and alleviated tissue damage, as shown by histological analysis. Survival analysis indicated that 4-OI post-sepsis treatment improved the overall survival rate in LPS-induced ALI mice.
Conclusion: 4-OI protects against sepsis-induced vascular injury and tissue damage by suppressing endothelial inflammation, oxidative stress, and preserving endothelial barrier integrity.
Keywords: 4-Octyl Itaconate; Acute lung injury; Endothelial cell; Sepsis; Vascular injury.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and consent to participate: All the animal procedures were approved by the Institutional Animal Care and Use Committee of Southwest Medical University and conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Ethical approval was granted by the Animal Experiment Ethics Committee of Southwest Medical University (No. 20221118-037). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4(7):499–511. - PubMed
-
- Bambouskova M, Gorvel L, Lampropoulou V, Sergushichev A, Loginicheva E, Johnson K, Korenfeld D, Mathyer ME, Kim H, Huang LH, Duncan D, Bregman H, Keskin A, Santeford A, Apte RS, Sehgal R, Johnson B, Amarasinghe GK, Soares MP, Satoh T, Akira S, Hai T, de Strong G, Auclair C, Roddy K, Biller TP, Jovanovic SA, Klechevsky M, Stewart E, Randolph KM, Artyomov GJ. Electrophilic properties of Itaconate and derivatives regulate the IkappaBzeta-ATF3 inflammatory axis. Nature. 2018;556(7702):501–504. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

