Targeting neuroinflammation: 3-monothiopomalidomide a new drug candidate to mitigate traumatic brain injury and neurodegeneration
- PMID: 40524167
- PMCID: PMC12172326
- DOI: 10.1186/s12929-025-01150-w
Targeting neuroinflammation: 3-monothiopomalidomide a new drug candidate to mitigate traumatic brain injury and neurodegeneration
Abstract
Background: Traumatic Brain Injury (TBI) is a major risk factor for neurodegenerative disorders such as Parkinson's disease (PD) and Alzheimer's disease (AD), with neuroinflammation playing a critical role in the secondary cell death that exacerbates the initial injury. While targeting neuroinflammation holds significant therapeutic promise, clinical trials of available anti-inflammatory agents have fallen short. 3-Mono-thiopomalidomide (3-MP), a novel immunomodulatory imide drug (IMiD), was designed to curb inflammation without the adverse effects of traditional IMiDs and was evaluated across models involving neuroinflammation.
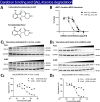
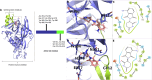
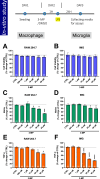
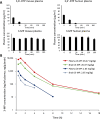
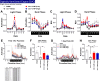
Methods: 3-MP anti-inflammatory activity was evaluated across cellular (RAW 264.7, IMG cells) and mouse studies following lipopolysaccharide (LPS)-challenge (for pro- and anti-inflammatory cytokines/chemokines), and mice subjected to controlled cortical impact (CCI) moderate traumatic brain injury (TBI). 3-MP human cereblon binding, including neosubstrate and molecular modeling evaluation, as well as chicken teratogenicity, ex vivo mouse and human stability studies, and mouse pharmacokinetics were appraised.
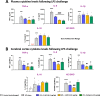
Results: 3-MP binds human cereblon, a key protein in the E3 ubiquitin ligase complex, without triggering downstream cascades leading to thalidomide-like teratogenicity in chicken embryos. 3-MP reduces pro-inflammatory markers in LPS-stimulated mouse macrophage and microglial cell cultures, and lowers pro-inflammatory cytokine/chemokine levels in plasma and brain of mice challenged with systemic LPS without lowering anti-inflammatory IL-10. 3-MP readily enters brain following systemic administration, and achieves a brain/plasma concentration ratio of 0.44-0.47. 3-MP mitigates behavioral impairments and reduces activation of astrocytes and microglia in mice challenged with CCI TBI.
Conclusion: 3-MP represents a promising new class of thalidomide-like IMiDs with potent anti-inflammatory effects that offers potential for treating TBI and possibly other neurodegenerative diseases possessing a prominent neuroinflammatory component.
Keywords: Cereblon; Immunomodulatory imide drugs (IMiDs); Microglia; Neurodegeneration; Neuroinflammation; Pomalidomide; Spalt like transcription factor 4 (SALL4); Teratogenicity; Traumatic brain injury.
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This is a preclinical (non-clinical) research study that does not require ethics approval and consent to participate for human subjects. All preclinical animal studies were undertaken on fully approved Institutional Animal Care and Use Committee protocols (see Materials and Methods). Consent for publication: Not applicable (our manuscript does not contain any individual person’s data in any form). Competing interests: DSK, YKK, SK, MYL, JK and IH are employed by and have stock in Aevis Bio Inc., which has patent coverage on 3-MP. All other authors declare a lack of competing of interest.
Figures





References
-
- Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung YC, Punchak M, Agrawal A, Adeleye AO, Shrime MG, Rubiano AM, Rosenfeld JV, Park KB. Estimating the global incidence of traumatic brain injury. J Neurosurg. 2018;130(4):1080–97. - PubMed
-
- Tagliaferri F, Compagnone C, Korsic M, Servadei F, Kraus J. A systematic review of brain injury epidemiology in Europe. Acta Neurochir (Wien). 2006;148(3):255–68. - PubMed
-
- Shi HY, Wang SN, Lee KT. Temporal trends and volume-outcome associations in periampullary cancer patients: a propensity score-adjusted nationwide population-based study. Am J Surg. 2014;207(4):512–9. - PubMed
MeSH terms
Substances
Grants and funding
- K01 AG000994/AG/NIA NIH HHS/United States
- RS-2024-0034124913/Ministry of Health & Welfare and Ministry of Science and ICT, Republic of Korea
- KDDF RS-2024-00338017/Korea Drug Development Fund funded by the Ministry of Science and ICT, Ministry of Trade, Industry, and Energy, and Ministry of Health and Welfare, Republic of Korea
- AG000994/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

