Immune cell aberrations in Systemic Lupus Erythematosus: navigating the targeted therapies toward precision management
- PMID: 40524185
- PMCID: PMC12172322
- DOI: 10.1186/s11658-025-00749-z
Immune cell aberrations in Systemic Lupus Erythematosus: navigating the targeted therapies toward precision management
Abstract
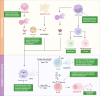
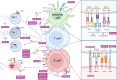
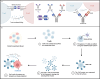
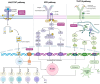
Systemic lupus erythematosus (SLE) is a complex autoimmune disease characterized by multilayered dysregulation of immune cell homeostasis, spanning B cell effector dysfunction, T follicular helper (Tfh) cell hyperactivity, and plasmacytoid dendritic cell (pDC) tolerance breakdown. Advances in high-parameter immunophenotyping, single-cell multiomics profiling, and spatial multiomics have redefined SLE pathogenesis, revealing stage-specific immune network perturbations. These discoveries have propelled mechanism-driven therapeutic strategies, including CD19-targeted chimeric antigen receptor T cell (CAR-T) therapy for B cell depletion, disruption of T-B cell synaptic signaling (CD40L inhibitors), and restoration of pDC tolerance (anti-BDCA2 antibodies). While patient heterogeneity poses challenges for universal therapeutic efficacy, emerging strategies integrating molecular endotyping and cellular biomarkers hold promise for overcoming these limitations. By aligning targeted therapies with the immunophenotypic signatures of individual patients, precision medicine approaches are expected to optimize treatment efficacy, minimize off-target effects, and ultimately enhance long-term clinical outcomes in SLE. This review synthesizes current insights into how immune cell perturbations contribute to SLE pathogenesis, modulate disease flares, and determine therapeutic refractoriness, with a critical synthesis of recent clinical trial outcomes.
Keywords: Immune cells; Immune dysregulation; Precise therapy; Systemic lupus erythematosus; Therapeutic targets.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. This manuscript does not contain any studies with human participants or animals performed by any of the authors. Consent for publication: Not applicable. This manuscript does not include details, images, or videos relating to an individual person. Competing interests: The authors declare that they have no competing interests.
Figures
Similar articles
-
B cell-targeted therapies in systemic lupus erythematosus: Current status and perspectives.Biochem Pharmacol. 2025 Sep;239:117018. doi: 10.1016/j.bcp.2025.117018. Epub 2025 Jun 3. Biochem Pharmacol. 2025. PMID: 40473226 Review.
-
Mesenchymal stem cell transplantation may be able to induce immunological tolerance in systemic lupus erythematosus.Biomed J. 2024 Aug;47(4):100724. doi: 10.1016/j.bj.2024.100724. Epub 2024 Apr 13. Biomed J. 2024. PMID: 38616015 Free PMC article. Review.
-
Clonal relationships between Tph and Tfh cells in patients with SLE and in murine lupus.bioRxiv [Preprint]. 2025 Jan 29:2025.01.27.635189. doi: 10.1101/2025.01.27.635189. bioRxiv. 2025. PMID: 39974998 Free PMC article. Preprint.
-
Chimeric Antigen Receptor T-cell therapy in systemic autoimmune rheumatic diseases: current insights and future prospects.J Rheum Dis. 2025 Jul 1;32(3):154-165. doi: 10.4078/jrd.2024.0122. Epub 2025 Jan 20. J Rheum Dis. 2025. PMID: 40584766 Free PMC article. Review.
-
Organ-based characterization of B cells in patients with systemic lupus erythematosus.Front Immunol. 2025 Jan 23;16:1509033. doi: 10.3389/fimmu.2025.1509033. eCollection 2025. Front Immunol. 2025. PMID: 39917309 Free PMC article.
References
-
- Siegel CH, Sammaritano LR. Systemic Lupus erythematosus: a review. JAMA. 2024;331(17):1480–91. - PubMed
-
- Kiriakidou M, Ching CL. Systemic Lupus Erythematosus. Ann Intern Med. 2020;172 (11):Itc81-itc96. - PubMed
-
- Caielli S, Wan Z, Pascual V. Systemic Lupus erythematosus pathogenesis: interferon and beyond. Annu Rev Immunol. 2023;41:533–60. - PubMed
-
- Wu Y, Zhao M, Gong N, Zhang F, Chen W, Liu Y. Immunometabolomics provides a new perspective for studying systemic lupus erythematosus. Int Immunopharmacol. 2023;118: 109946. - PubMed
-
- Tsokos GC. The immunology of systemic lupus erythematosus. Nat Immunol. 2024;25(8):1332–43. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

