Whole-lesion-aware network based on freehand ultrasound video for breast cancer assessment: a prospective multicenter study
- PMID: 40524235
- PMCID: PMC12172377
- DOI: 10.1186/s40644-025-00892-y
Whole-lesion-aware network based on freehand ultrasound video for breast cancer assessment: a prospective multicenter study
Abstract
Background: The clinical application of artificial intelligence (AI) models based on breast ultrasound static images has been hindered in real-world workflows due to operator-dependence of standardized image acquisition and incomplete view of breast lesions on static images. To better exploit the real-time advantages of ultrasound and more conducive to clinical application, we proposed a whole-lesion-aware network based on freehand ultrasound video (WAUVE) scanning in an arbitrary direction for predicting overall breast cancer risk score.
Methods: The WAUVE was developed using 2912 videos (2912 lesions) of 2771 patients retrospectively collected from May 2020 to August 2022 in two hospitals. We compared the diagnostic performance of WAUVE with static 2D-ResNet50 and dynamic TimeSformer models in the internal validation set. Subsequently, a dataset comprising 190 videos (190 lesions) from 175 patients prospectively collected from December 2022 to April 2023 in two other hospitals, was used as an independent external validation set. A reader study was conducted by four experienced radiologists on the external validation set. We compared the diagnostic performance of WAUVE with the four experienced radiologists and evaluated the auxiliary value of model for radiologists.
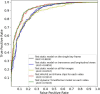
Results: The WAUVE demonstrated superior performance compared to the 2D-ResNet50 model, while similar to the TimeSformer model. In the external validation set, WAUVE achieved an area under the receiver operating characteristic curve (AUC) of 0.8998 (95% CI = 0.8529-0.9439), and showed a comparable diagnostic performance to that of four experienced radiologists in terms of sensitivity (97.39% vs. 98.48%, p = 0.36), specificity (49.33% vs. 50.00%, p = 0.92), and accuracy (78.42% vs.79.34%, p = 0.60). With the WAUVE model assistance, the average specificity of four experienced radiologists was improved by 6.67%, and higher consistency was achieved (from 0.807 to 0.838).
Conclusion: The WAUVE based on non-standardized ultrasound scanning demonstrated excellent performance in breast cancer assessment which yielded outcomes similar to those of experienced radiologists, indicating the clinical application of the WAUVE model promising.
Keywords: Artificial intelligence; Breast neoplasms; Deep learning; Diagnosis; Ultrasonography; Video.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This multicenter study was divided into two parts: model development using a retrospective dataset and model validation using a prospective dataset. The retrospective study was approved by Ethics Committee of National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (No. 22/386-3588) with a waiver granted for the requirement of informed consent. The prospective study was approved by Ethics Committee of National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (No. 21/328-2999), and informed consent was obtained from each patient or their guardian before the US examination. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
External Validation of an Upgraded AI Model for Screening Ileocolic Intussusception Using Pediatric Abdominal Radiographs: Multicenter Retrospective Study.J Med Internet Res. 2025 Jul 8;27:e72097. doi: 10.2196/72097. J Med Internet Res. 2025. PMID: 40653922 Free PMC article.
-
Application Value of Deep Learning-Based AI Model in the Classification of Breast Nodules.Br J Hosp Med (Lond). 2025 Jun 25;86(6):1-19. doi: 10.12968/hmed.2025.0078. Epub 2025 Jun 15. Br J Hosp Med (Lond). 2025. PMID: 40554435
-
A multicenter diagnostic study of thyroid nodule with Hashimoto's thyroiditis enabled by Hashimoto's thyroiditis nodule-artificial intelligence model.Eur Radiol. 2025 Aug;35(8):4610-4620. doi: 10.1007/s00330-025-11422-6. Epub 2025 Feb 13. Eur Radiol. 2025. PMID: 39939425
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
References
-
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–63. - PubMed
-
- McBee MP, Awan OA, Colucci AT, Ghobadi CW, Kadom N, Kansagra AP, et al. Deep Learning in radiology. Acad Radiol. 2018;25:1472–80. - PubMed
Publication types
MeSH terms
Grants and funding
- No. 82171967/National Natural Science Foundation of China
- No. 2022-PUMCH-B-065/National High-level Hospital Clinical Research Funding
- No. 2022-PUMCH-B-066/National High-level Hospital Clinical Research Funding
- No. 2024-2-40210/Capital's Funds for Health Improvement and Research
- No. ZZ2024A10/Beijing Hope Run Special Fund of Cancer Foundation of China
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

