A repeated cross-sectional analysis of SARS-CoV-2 seroprevalence in Manila, the Philippines after implementation of the national COVID-19 vaccination program
- PMID: 40524246
- PMCID: PMC12168273
- DOI: 10.1186/s41182-025-00767-9
A repeated cross-sectional analysis of SARS-CoV-2 seroprevalence in Manila, the Philippines after implementation of the national COVID-19 vaccination program
Abstract
Background: SARS-CoV-2 seroepidemiological studies, which have been used to describe population-level immunity, are limited in the Philippines, despite the protracted course of the epidemic in the country. We follow-up on our previous work and aimed to estimate SARS-CoV-2 seroprevalence and infection rate among outpatient clinic attendees in Metro Manila, a year after the implementation of the national COVID-19 vaccination program.
Methods: We conducted four repeated cross-sectional surveys at the outpatient department of San Lazaro Hospital between March 2022 and January 2023. We performed χ2 test and analysis of variance to assess the differences in characteristics across different data collection periods.
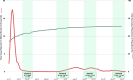
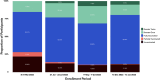
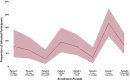
Results: A total of 765 participants were enrolled, ranging from 170 to 200 per period. Participant demographic, socioeconomic, and medical history were comparable across all data collection periods. Between March and October 2022, the proportion of participants who received a vaccine or booster dose significantly increased, from 77.9% to 90%. Seroprevalence across all data collection periods was consistently high, ranging from 97.8% to 99.5%. However, the geometric mean concentration of antibodies was highest in the data collection period following the Omicron-dominant wave. Infection rates were comparably low (< 10%) across periods, except for a peak at 16.7% in September to October 2022, which followed the rise in reported cases in Metro Manila.
Conclusion: Population-level seroprevalence among clinic attendees in Manila was consistently high a year after implementation of the national COVID-19 vaccination program, but analyses of antibody concentrations showed potential waning within a 3-month period.
Keywords: COVID-19; Philippines; SARS-CoV-2; Seroepidemiological study; Vaccination.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- Almendro-Vázquez P, Laguna-Goya R, Ruiz-Ruigomez M, Utrero-Rico A, Lalueza A, de la Calle GM, et al. Longitudinal dynamics of SARS-CoV-2-specific cellular and humoral immunity after natural infection or BNT162b2 vaccination. PLoS Pathog. 2021;17: e1010211. 10.1371/journal.ppat.1010211. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
