Independent validation and outlier analysis of EuroPOND alzheimer's disease staging model using ADNI and real-world clinical data
- PMID: 40524264
- PMCID: PMC12172318
- DOI: 10.1186/s13195-025-01788-6
Independent validation and outlier analysis of EuroPOND alzheimer's disease staging model using ADNI and real-world clinical data
Abstract
Background: Event-based modeling (EBM) traces sequential progression of events in complex processes like neurodegenerative diseases, adept at handling uncertainties. This study validated an EBM for Alzheimer's disease (AD) staging designed by EuroPOND, an EU-funded Horizon 2020 project, using research and real-world datasets, a crucial step towards application in multi-center trials.
Methods: The training dataset comprised 1737 subjects from ADNI-1/GO/2, using the EuroPOND EBM toolbox. Testing datasets included a research cohort from University of Antwerp (controls, CN (n = 46), subjective cognitive decline, SCD (n = 10), mild cognitive impairment, MCI (n = 47), AD dementia, ADD (n = 16)) and a real-world cohort from 9 Belgian Dementia Council memory clinics (CN (n = 91), SCD (n = 66), (non-amnestic) naMCI (n = 54), aMCI (n = 255), and ADD (n = 220). Biomarkers included: 2 clinical scores (Mini Mental State Examination (MMSE), Rey Auditory Verbal Learning Test (RAVLT)); 3 CSF-biomarkers (Aβ1-42, P-tau181, total-Tau); and 4 magnetic resonance imaging (MRI) biomarkers (volumes of the hippocampi, temporal, parietal, and frontal cortices) computed with icobrain dm. The naMCI and aMCI groups were compared by EBM stage proportions, and the model's effectiveness at patient level was evaluated.
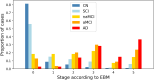
Results: The research cohort's maximum likelihood event sequence comprised CSF Aβ1-42, P-tau181, T-tau, RAVLT, MMSE, and cortical volumes. The clinical cohort's order was frontal cortex volume, MMSE, and remaining cortical regions. aMCI subjects showed higher staging than naMCI, with 54% in the two most advanced stages compared to 38% in naMCI. In the research cohort, 10 outliers were identified with potential mismatches between assigned stages and clinical or biomarker profiles, with CN (n = 4) and SCD (n = 2) subjects assigned in stage 4, one control in stage 9 with abnormal imaging, and three aMCI cases in stage 0 despite clinical or volumetric signs of impairment.
Conclusions: This study highlights the generalizability of EuroPOND's AD EBM model across research and real-world clinical datasets, supporting its use in multi-center trials. aMCI subjects generally reside in more advanced stages than naMCI, who may not necessarily have AD, demonstrating utility for precision recruitment/screening.
Keywords: Alzheimer’s disease; Automated volumetry; Biomarkers; Event-based modelling; Magnetic resonance imaging.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The REMEMBER study was approved by the ethics committee of University of Antwerp / Universitair Ziekenhuis Antwerpen, Antwerp (N°16/2/18) and by the ethics committees of Algemeen Ziekenhuis Sint-Jan Brugge-Oostende, Brugge (N°1992); Centre Hospitalier Universitaire Brugmann (CHU Brugmann), Brussels (N°2016/84); Centre Hospitalier Universitaire Liège (CHU Liège), Liège (N°2012/274); Cliniques Universitaires de Bruxelles (ULB), Hôpital Erasme, Brussels (N°P2016/187); Cliniques Universitaires Saint-Luc (UCL), Brussels (N°2016/07jui/261); Cliniques St-Pierre Ottignies, Ottignies (N°OM045); Universitair Ziekenhuis Brussel, Brussels (N°2016/183); and Ziekenhuis Netwerk Antwerp (ZNA), Antwerp (N°4730). The research was conducted in accordance with the Declaration of Helsinki and informed written consent was obtained from all participants from the University of Antwerp prospective research cohort, that was approved by the ethics committee of University of Antwerp / Universitair Ziekenhuis Antwerpen, Antwerp (N°15/38/394). icobrain dm is a proprietary software, developed by icometrix for the automated quantification of brain volumes and white matter hyperintensities. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



References
-
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s association workgroups on diagnostic guidelines for alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–92. - PMC - PubMed
-
- Blennow K, Zetterberg H. Biomarkers for alzheimer’s disease: current status and prospects for the future. J Intern Med. 2018;284(6):643–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- herinneringen/Interreg V programme Flanders-The Netherlands of the European Regional Development Fund (ERDF)
- herinneringen/Interreg V programme Flanders-The Netherlands of the European Regional Development Fund (ERDF)
- herinneringen/Interreg V programme Flanders-The Netherlands of the European Regional Development Fund (ERDF)
LinkOut - more resources
Full Text Sources
Medical

