Release of Bisphenol A from Dental Materials: Risks and Future Perspectives
- PMID: 40524375
- PMCID: PMC12301515
- DOI: 10.1177/00220345251337728
Release of Bisphenol A from Dental Materials: Risks and Future Perspectives
Abstract
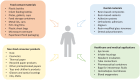
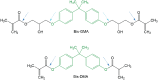
The gradual phaseout of dental amalgam has contributed to a significant increase in the use of resin-based materials. While these materials offer several desirable properties, concerns persist regarding their biocompatibility, particularly the release of bisphenol A (BPA). BPA is an endocrine-disrupting chemical linked to adverse effects on human health, including reproductive, developmental, and metabolic disorders. Although food contact materials are the primary source of human BPA exposure and the contribution of dental materials is minor, the associated risks cannot be dismissed due to BPA's nonmonotonic dose-response relationship. In 2023, the European Food Safety Authority proposed a 20,000-fold reduction in the tolerable daily intake of BPA to 0.2 ng/kg body weight, citing immune system effects at extremely low doses. This proposal has sparked regulatory and scientific debate, as adopting such a stringent limit would effectively ban the use of BPA in food contact materials and many other products. Given this context, it is essential to assess the release of BPA from dental materials both in vitro and in vivo. However, data interpretation is complicated by methodological inconsistencies, including variations in material composition, specimen preparation, choice of extraction media, experimental duration, and analytical methods. In addition, pivotal differences in reporting results make it difficult to synthesize findings and draw reliable conclusions. This review examines the controversy surrounding BPA, critically evaluates evidence on its release from dental materials, and explores mitigation strategies. By highlighting gaps in knowledge and proposing future research directions, this review aims to provide clinicians, researchers, and policymakers with a clearer understanding of BPA-related complexities, ultimately contributing to patient safety and material innovation.
Keywords: biocompatibility; composite materials; endocrinology; resin(s); sealants; toxicology.
Conflict of interest statement
Declaration of Conflicting InterestsThe authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

References
-
- Arenholt-Bindslev D, Breinholt V, Preiss A, Schmalz G. 1999. Time-related bisphenol-A content and estrogenic activity in saliva samples collected in relation to placement of fissure sealants. Clin Oral Investig. 3(3):120–125. - PubMed
-
- Atkinson JC, Diamond F, Eichmiller F, Selwitz R, Jones G. 2002. Stability of bisphenol A, triethylene-glycol dimethacrylate, and bisphenol A dimethacrylate in whole saliva. Dent Mater. 18(2):128–135. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

