Four Cases of Prurigo Nodularis Requiring Nemolizumab due to Persistent Pruritus or Lesions after Dupilumab Treatment
- PMID: 40524402
- PMCID: PMC12186433
- DOI: 10.2340/actadv.v105.43419
Four Cases of Prurigo Nodularis Requiring Nemolizumab due to Persistent Pruritus or Lesions after Dupilumab Treatment
Conflict of interest statement
YM and KI have received lecture fees from Maruho Co., which markets nemolizumab.
Figures
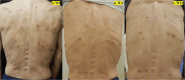
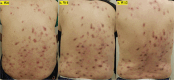
Similar articles
-
Trial of Nemolizumab in Moderate-to-Severe Prurigo Nodularis.N Engl J Med. 2020 Feb 20;382(8):706-716. doi: 10.1056/NEJMoa1908316. N Engl J Med. 2020. PMID: 32074418 Clinical Trial.
-
Efficacy and safety of nemolizumab and topical corticosteroids for prurigo nodularis: results from a randomized double-blind placebo-controlled phase II/III clinical study in patients aged ≥ 13 years.Br J Dermatol. 2024 Jul 16;191(2):200-208. doi: 10.1093/bjd/ljae131. Br J Dermatol. 2024. PMID: 38629497 Clinical Trial.
-
A critical evaluation of nemolizumab for prurigo nodularis.Expert Rev Clin Immunol. 2024 Jun;20(6):577-587. doi: 10.1080/1744666X.2024.2306225. Epub 2024 Jan 19. Expert Rev Clin Immunol. 2024. PMID: 38217530 Review.
-
Phase 3 Trial of Nemolizumab in Patients with Prurigo Nodularis.N Engl J Med. 2023 Oct 26;389(17):1579-1589. doi: 10.1056/NEJMoa2301333. N Engl J Med. 2023. PMID: 37888917 Clinical Trial.
-
Nemolizumab: An Innovative Biologic Treatment to Control Interleukin 31, a Key Mediator in Atopic Dermatitis and Prurigo Nodularis.Actas Dermosifiliogr. 2022 Jul-Aug;113(7):674-684. doi: 10.1016/j.ad.2021.12.014. Epub 2021 Dec 23. Actas Dermosifiliogr. 2022. PMID: 35842249 Review. English, Spanish.
References
-
- Pereira MP, Steinke, S, Zeidler C, Forner C, Riepe C, Augustin M, et al. European Academy of Dermatology and Venereology European prurigo project: expert consensus on the definition, classification and terminology of chronic prurigo. J Eur Acad Dermatol Venereol 2018; 32: 1059-1065. 10.1111/jdv.14570 - DOI - PubMed
-
- Leis M, Fleming P, Lynde CW. Prurigo nodularis: review and emerging treatments. Skin Therapy Lett 2021; 26: 5–8. - PubMed
LinkOut - more resources
Full Text Sources

