Short-Term Severe Energy Restriction Promotes Molecular Health and Reverses Aging Signatures in Adults With Prediabetes in the PREVIEW Study
- PMID: 40524498
- PMCID: PMC12341815
- DOI: 10.1111/acel.70123
Short-Term Severe Energy Restriction Promotes Molecular Health and Reverses Aging Signatures in Adults With Prediabetes in the PREVIEW Study
Abstract
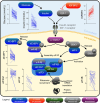
Prediabetes, characterized by impaired fasting glucose and/or glucose tolerance, is associated with organ damage, increased mortality, and accelerated aging, even before diabetes onset. Severe short-term energy restriction while maintaining essential nutrient intake is among the most effective strategies for weight loss, metabolic health improvement, and delaying type 2 diabetes progression. Extracellular vesicles contribute to these metabolic benefits; however, the impact of energy-restriction-induced weight loss on the extracellular vesicle proteome remains incompletely understood. This study employed targeted and untargeted proteomics to investigate the effect of an 8-week severely energy-restricted diet on the plasma proteome in adults with prediabetes from Sydney, Australia, as part of the PREVIEW study. Circulating extracellular vesicles were enriched in plasma using an immunoaffinity-based protocol. A total of 44 participants who achieved at least a 12% weight loss and provided informed consent were included in the study. Paired changes in over 2000 proteins between baseline and week 8 were analyzed. Following the intervention, multiple proteins associated with inflammation and senescence were significantly reduced, reversing the increase commonly associated with aging. The decline in inflammatory and senescence markers may have been mediated by extracellular vesicles, as indicated by significantly lower circulating levels of several vesicular markers. Additionally, several markers of protein synthesis downstream of mTORC1 and protein degradation were significantly reduced in vesicle-enriched plasma, suggesting decreased intercellular secretion and/or trafficking. Overall, this study identifies a diet-induced proteomic signature suggestive of reduced inflammation, lower senescence, and enhanced vesicle-associated proteostasis, potentially conferring health benefits beyond glycemic control.
© 2025 The Author(s). Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
L.F. and J.B.M. are authors of widely recognized nutrition books on metabolic health. The remaining authors declare no conflicts of interest. This article was not commissioned, and the funding sources had no role in the study design, data interpretation, or the generation of the findings. No author received financial or other benefits for their contributions to this work.
Figures



References
Further Reading
-
- Kassambara, A. 2018. Ggpubr: ‘ggplot2’ Based Publication Ready Plots. R package version
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

