The effects of a nurse-led HAPA-based discharge planning on post-operative outcomes in urolithiasis patients with double-J stents: protocol for a randomized controlled trial
- PMID: 40524532
- PMCID: PMC12175186
- DOI: 10.1080/07853890.2025.2519676
The effects of a nurse-led HAPA-based discharge planning on post-operative outcomes in urolithiasis patients with double-J stents: protocol for a randomized controlled trial
Abstract
Introduction: Patients with urolithiasis undergoing double-J stent placement often face challenges during the post-operative period, including insufficient discharge preparedness and difficulties in self-management. Discharge planning can assist patients during this critical period. However, the effects of delivering a nurse-led discharge planning program remain under-studied. This study aims to apply the Health Action Process Approach (HAPA) model to develop and evaluate a nurse-led discharge planning intervention.
Aim: This randomized controlled trial evaluates the effectiveness of a nurse-led, HAPA-based discharge planning intervention in improving discharge preparedness, self-management behaviors, and quality of life in patients undergoing double-J stent placement for urolithiasis.
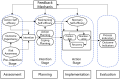
Methods: A total of 86 patients with urolithiasis undergoing double-J stent placement will be randomly assigned to either the intervention group (HAPA-based discharge planning + usual care planning) or the control group (usual care planning). The intervention consists of four phases: assessment, planning, implementation, and evaluation, delivered during hospitalization, with a 30-day follow-up post-discharge. Primary outcomes include discharge readiness, self-management ability, and quality of life, which will be assessed at baseline, discharge, and 7 and 30 days post-discharge. Secondary outcomes include complications following double-J stent placement and unplanned healthcare service utilization rate.
Discussion: The results of this study will provide empirical evidence supporting the application of the HAPA model in post-operative discharge planning for urolithiasis patients. If successful, this intervention could lead to significant improvements in self-management behaviors, reduce complications and unplanned healthcare service utilization rates, and ultimately enhance the quality of life for these patients.
Trial registration number: Registered in the Chinese Clinical Trial Registry (ChiCTR2400093503).
Version identifier: Version 1.0, 2024-11-07.
Keywords: Health Action Process Approach; Public health; discharge planning; double-J tube; protocols; randomised controlled trial (RCT); readiness for discharge; self-management; urolithiasis.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources