Real-world clinical burden of patients presenting with vaginitis symptoms in the United States
- PMID: 40524705
- PMCID: PMC12167788
- DOI: 10.1016/j.xagr.2025.100504
Real-world clinical burden of patients presenting with vaginitis symptoms in the United States
Abstract
Background: While vaginitis is a leading cause of primary care visits among women with a gynecologic-related diagnosis, there are limited contemporary data on the healthcare burden. This study describes the real-world healthcare resource utilization (HCRU) of patients presenting with vaginitis symptoms in the United States (US) at symptom presentation and over long-term follow-up.
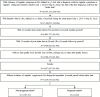
Methods: This retrospective study utilized IQVIA's Longitudinal Prescription (LRx) and Medical Claims (Dx) databases to capture patients presenting with vaginitis symptoms from January 1, 2018 to September 30, 2022. The date of the first diagnosis code for vaginitis or related symptoms was considered the first clinical presentation ("index visit"). Healthcare visits, diagnostic testing, and treatments were assessed for patients at presentation (index date +2 days) and 12-month follow-up, stratified by pregnancy status at index. In a subset of patients with linkage to IQVIA Ambulatory EMR - US (AEMR), multivariable models were used to evaluate associations between insurance type, patient characteristics, diagnostic test(s) performed at presentation, and HCRU outcomes (subsequent vaginitis-related healthcare visits and ≥2 vaginitis treatment dates) over follow-up.
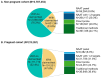
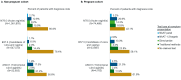
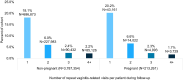
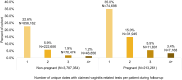
Results: A total of 18,745,351 people were documented with vaginitis symptoms or vaginitis in the study selection window, of which 4,000,615 patients met all selection criteria for analysis: 3,787,354 were not pregnant and 213,261 had evidence of pregnancy. About one-fourth (23.8%) of the non-pregnant cohort and half (47.6%) of the pregnant cohort had claims for at least 1 diagnostic test at symptom presentation, with traditional methods being most commonly used (44.1% and 36.4% for non-pregnant and pregnant patients, respectively), followed by direct probe (20.0% and 24.1%), and lastly nucleic acid amplification test (NAAT) panel (including bacterial vaginosis, vulvovaginal candidiasis, and trichomoniasis; 6.6% and 8.3%). Despite low diagnostic testing rates, 50.1% of the non-pregnant and 60.9% of the pregnant cohort received prescribed vaginitis treatment, most frequently metronidazole or fluconazole, and 28.8% of the non-pregnant and 30.9% of the pregnant cohort had subsequent vaginitis-related visits within 12 months. Among both the non-pregnant and pregnant cohorts, patients with Medicaid insurance had significantly higher odds of repeat healthcare visits and ≥2 treatment dates during follow-up relative to patients with commercial insurance.
Conclusion: This study demonstrated that vaginitis poses a high clinical burden in the US, possibly attributed to low diagnostic testing rates, use of tests with poor performance, and high rates of empiric treatment. There is an unmet need for rapid, accurate vaginitis diagnostic testing at the point-of-care to reduce empiric prescribing and improve diagnostic and treatment accuracy and efficiency.
Keywords: NAAT; diagnostics; healthcare utilization; infectious disease; vaginitis; vaginosis.
© 2025 The Authors.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous

