GSTP1 knockdown induces metabolic changes affecting energy production and lipid balance in pancreatic cancer cells
- PMID: 40524738
- PMCID: PMC12169041
- DOI: 10.1080/23723556.2025.2518773
GSTP1 knockdown induces metabolic changes affecting energy production and lipid balance in pancreatic cancer cells
Abstract
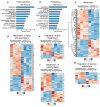
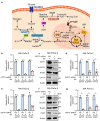
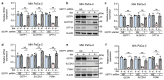
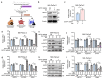
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive cancer with limited treatment options, underscoring the need for novel therapeutic targets. Metabolic reprogramming is a hallmark of PDAC, enabling tumor cells to sustain rapid proliferation and survive under nutrient-deprived conditions. While glutathione S-transferase pi 1 (GSTP1) is a known regulator of redox homeostasis in PDAC, its role in metabolic adaptation remains unclear. Here, we show that GSTP1 knockdown disrupts PDAC metabolism, leading to downregulation of key metabolic enzymes (ALDH7A1, CPT1A, SLC2A3, PGM1), ATP depletion, mitochondrial dysfunction, and phospholipid remodeling. Phospholipid remodeling, including an increase in phosphatidylcholine (PC) levels, further suggests a compensatory response to metabolic stress. Importantly, GSTP1 knockdown led to elevated lipid peroxidation, increasing 4-hydroxynonenal (4-HNE) accumulation. Treatment with the antioxidant N-acetyl cysteine (NAC) partially restored metabolic gene expression, reinforcing GSTP1's role in the interplay between redox regulation and metabolism in PDAC. By disrupting multiple metabolic pathways, GSTP1 depletion creates potential therapeutic vulnerabilities that could be targeted through metabolic and oxidative stress-inducing therapies to enhance treatment efficacy.
Keywords: Pancreatic ductal adenocarcinoma; glutathione S-transferase pi 1 (GSTP1); metabolic reprogramming; metabolomics; therapeutic targeting.
© 2025 The Author(s). Published with license by Taylor & Francis Group, LLC.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
