Sleep Fragmentation as a Diagnostic Biomarker of Traumatic Brain Injury
- PMID: 40524781
- PMCID: PMC12167842
- DOI: 10.1089/neur.2025.0050
Sleep Fragmentation as a Diagnostic Biomarker of Traumatic Brain Injury
Abstract
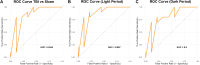
Sleep disturbances are among the most prevalent and persistent consequences of traumatic brain injury (TBI), yet they remain underutilized as clinical indicators of injury status. In this perspective, we propose that sleep fragmentation-defined as the frequency of transitions between sleep and wakefulness-represents a functional, scalable, and underrecognized diagnostic biomarker of TBI. Drawing on empirical findings from a mouse model of diffuse TBI, we show that summary measures of sleep fragmentation and duration can reliably distinguish injured from uninjured animals using dimensionality reduction and machine learning techniques. Current biomarkers such as glial fibrillary acidic protein and neurofilament light chain provide valuable insights into structural damage but offer limited information about how injury affects behavior and day-to-day function. Sleep-based metrics, by contrast, reflect neural network integrity and capture ongoing physiological disruption. Critically, these metrics can be collected non-invasively, longitudinally, and in real-world settings using actigraphy, making them a practical complement to blood-based diagnostics that require biological sampling and specialized laboratory infrastructure. Our analysis demonstrates that sleep metrics collected over 48 h post-injury-specifically the number of sleep-wake transitions-carry a strong diagnostic signal. Sleep metrics offer a behaviorally grounded complement aligned with the goals of precision medicine and functional assessment. With further validation, these features may also support monitoring recovery or stratifying injury severity. This perspective highlights sleep fragmentation as a non-invasive diagnostic biomarker for TBI with the potential to enhance individualized monitoring and support early detection efforts in both research and clinical settings.
Keywords: biomarker; concussion; mouse; sleep fragmentation; state transitions.
© The Author(s) 2025. Published by Mary Ann Liebert, Inc.
Figures



Similar articles
-
Impacts of traumatic brain injury severity and sex on sleep architecture, duration, and fragmentation.Neurobiol Sleep Circadian Rhythms. 2025 May 15;18:100127. doi: 10.1016/j.nbscr.2025.100127. eCollection 2025 May. Neurobiol Sleep Circadian Rhythms. 2025. PMID: 40495996 Free PMC article.
-
Structural brain network deviations predict recovery after traumatic brain injury.Neuroimage Clin. 2023;38:103392. doi: 10.1016/j.nicl.2023.103392. Epub 2023 Mar 30. Neuroimage Clin. 2023. PMID: 37018913 Free PMC article.
-
Plasma glial fibrillary acidic protein and neurofilament light chain, but not tau, are biomarkers of sports-related mild traumatic brain injury.Brain Commun. 2020 Sep 7;2(2):fcaa137. doi: 10.1093/braincomms/fcaa137. eCollection 2020. Brain Commun. 2020. PMID: 33543129 Free PMC article.
-
Exploring Serum Biomarkers for Mild Traumatic Brain Injury.In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 22. In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 22. PMID: 26269900 Free Books & Documents. Review.
-
Immune-endocrine interactions in the pathophysiology of sleep-wake disturbances following traumatic brain injury: A narrative review.Brain Res Bull. 2022 Jul;185:117-128. doi: 10.1016/j.brainresbull.2022.04.017. Epub 2022 May 7. Brain Res Bull. 2022. PMID: 35537569 Review.
References
-
- Leo P, McCrea M. Translational Research in Traumatic Brain Injury. CRC Press/Taylor and Francis Group: Boca Raton, FL; 2016. - PubMed
LinkOut - more resources
Full Text Sources
