Real-world uptake of nirsevimab, RSV maternal vaccine, and RSV vaccines for older adults: a systematic review and meta-analysis
- PMID: 40524797
- PMCID: PMC12167775
- DOI: 10.1016/j.eclinm.2025.103281
Real-world uptake of nirsevimab, RSV maternal vaccine, and RSV vaccines for older adults: a systematic review and meta-analysis
Abstract
Background: In clinical trials, recently introduced respiratory syncytial virus (RSV) immunisation products have shown high efficacy in preventing severe RSV outcomes. Implementing successful immunisation programmes is however key to realising these benefits in real-world settings. We aimed to investigate uptake of the long-acting monoclonal antibody nirsevimab, the RSV maternal vaccine, and RSV vaccines for older adults in countries where immunisation programmes have been introduced, and to explore how uptake varies between countries and population subgroups.
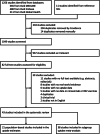
Methods: In this systematic review and meta-analysis, we carried out four monthly searches in Medline, Embase, and Global Health databases for studies reporting uptake of nirsevimab, the RSV maternal vaccine, and RSV vaccines for older adults. We included population-based studies published between December 1, 2022 and February 5, 2025. Two independent reviewers screened studies, extracted data, and completed a risk of bias assessment using the Joanna Briggs Institute (JBI) Critical Appraisal Tools. We assessed uptake stratified by country and socio-demographic and clinical subgroups. Meta-analyses were conducted using random-effects modelling. PROSPERO registration number: CRD42025643585.
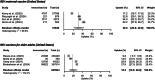
Findings: We screened a total of 1267 studies, 43 of which met the inclusion criteria reporting data on over 1.38 million individuals from six countries. Nirsevimab uptake data were reported in 34 studies: 16 from Spain, eight from the United States, seven from France, one with combined data from Andorra and Spain, and one from each of Italy and Luxembourg. Our pooled estimates showed that nirsevimab uptake on population level was 90.1% (95% confidence interval (CI): 86.4-92.9) in Spain and 51.2% (95% CI: 29.3-72.7) in the United States during the 2023/24 RSV season. Uptake data for the RSV maternal vaccine and RSV vaccines for older adults were reported in five and eight studies, respectively, all from the United States. Meta-analysis showed population-level uptake of 30.5% (95% CI: 20.6-42.6) and 18.2% (95% CI: 10.8-28.9), respectively. Uptake varied across subgroups.
Interpretation: Uptake of nirsevimab varied substantially between the countries that have implemented infant RSV immunisation programmes. Despite the limited number of studies and the lack of more accurate data at national level the low uptake estimates for RSV maternal vaccine and RSV vaccines for older adults are concerning. National, clinical, and public health initiatives are needed to increase uptake of RSV immunisation products and ensure maximum benefit to people currently at risk of severe RSV outcomes.
Funding: Health Data Research UK, Inflammation and Immunity Driver Programme.
Keywords: Immunisation programmes; Nirsevimab; RSV vaccines; Respiratory syncytial virus (RSV); Vaccine uptake.
© 2025 The Authors.
Conflict of interest statement
JS has participated in Sanofi advisory board on nirsevimab in 2022, and participated in Allergy Therapeutics advisory board on immunotherapy. JS has received research grant from UKRI Medical Research Council. JS has received support from ALK for attending meetings and/or travel. JB has received research funding from AstraZeneca, paid to his institution. AS acknowledges the support of Health Data Research UK. DT is funded by Health Data Research UK, Inflammation and Immunity Driver Programme. BL has received Asthma + Lung UK Early Career Researcher grant (WHRD_PB1062). BG has received the studentship award from the Health Data Research UK-The Alan Turing Institute Wellcome PhD Programme in Health Data Science (Grant Ref: 218529/Z/19/Z). SD has received investigator led grant from MSD in relation to RSV, paid to his institution. SD has received support from Sanofi for attending meetings and/or travel. SD has provided consultancy and/or investigator roles in relation to product development for Janssen, AstraZeneca, Pfizer, Moderna, Valneva, MSD, iLiAD, MundiPharma, and Sanofi, paid to his institution. SD is a member of the UK Department of Health and Social Care's (DHSC) Joint Committee on Vaccination and Immunisation (JCVI) RSV subcommittee and Medicines and Healthcare products Regulatory Agency's (MHRA) Paediatric Medicine Expert Advisory Group (PMEAG). TW has received research funding from Respiratory Syncytial Virus Consortium in Europe (RESCEU), Wellcome Trust, Imperial College London, and National Institute for Health Research. All other authors declare no competing interests.
Figures

Similar articles
-
Real-world effectiveness of nirsevimab against respiratory syncytial virus disease in infants: a systematic review and meta-analysis.Lancet Child Adolesc Health. 2025 Jun;9(6):393-403. doi: 10.1016/S2352-4642(25)00093-8. Epub 2025 May 1. Lancet Child Adolesc Health. 2025. PMID: 40319903
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Effectiveness and impact of universal prophylaxis with nirsevimab in infants against hospitalisation for respiratory syncytial virus in Galicia, Spain: initial results of a population-based longitudinal study.Lancet Infect Dis. 2024 Aug;24(8):817-828. doi: 10.1016/S1473-3099(24)00215-9. Epub 2024 Apr 30. Lancet Infect Dis. 2024. PMID: 38701823
-
The recent landscape of RSV vaccine research.Ther Adv Vaccines Immunother. 2025 Jan 10;13:25151355241310601. doi: 10.1177/25151355241310601. eCollection 2025. Ther Adv Vaccines Immunother. 2025. PMID: 39802673 Free PMC article. Review.
-
Early Estimate of Nirsevimab Effectiveness for Prevention of Respiratory Syncytial Virus-Associated Hospitalization Among Infants Entering Their First Respiratory Syncytial Virus Season - New Vaccine Surveillance Network, October 2023-February 2024.MMWR Morb Mortal Wkly Rep. 2024 Mar 7;73(9):209-214. doi: 10.15585/mmwr.mm7309a4. MMWR Morb Mortal Wkly Rep. 2024. PMID: 38457312 Free PMC article.
References
-
- Kampmann B., Madhi S.A., Munjal I., et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med. 2023;388(16):1451–1464. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

