Development of Human Skin Equivalents with Inducible Ceramide Depletion for In Vitro Modeling of Lipid Impairment
- PMID: 40524817
- PMCID: PMC12167793
- DOI: 10.1016/j.xjidi.2025.100383
Development of Human Skin Equivalents with Inducible Ceramide Depletion for In Vitro Modeling of Lipid Impairment
Abstract
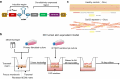
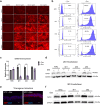
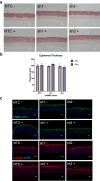
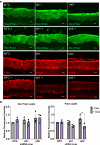
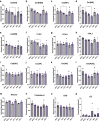
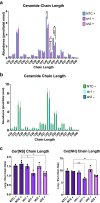
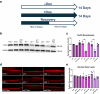
The lipid composition of the epidermis plays a critical role in the skin's barrier function, and defects in lipid synthesis or assembly can cause a spectrum of skin diseases, ranging from dry skin to severe ichthyoses. The aim of this study was to develop an in vitro model of human skin with tunable inhibition of lipid synthesis. Human N/TERT keratinocytes were engineered to express doxycycline-inducible short hairpin RNAs targeting ceramide synthase 3, which is essential for synthesis of ultralong-chain ceramides and skin barrier function. We show that 3-dimensional human skin equivalents with induced knockdown of ceramide synthase 3 display normal stratification and terminal differentiation but have reduced Nile red staining for polar lipids. Further analysis of the lipidome by mass spectrometry confirmed a significant reduction in specific classes of ceramides and ceramide chain length in the ceramide synthase 3-depleted human skin equivalents. We also show that ceramide synthase 3 knockdown is reversible upon removal of doxycycline and can be used to study recovery and repair of epidermal lipids. Together, these findings provide an overall strategy for genetically regulating the lipid composition within human skin models and establish a tunable in vitro model of ceramide deficiency.
Keywords: 3D model; Barrier function; CERS3; Ceramide; Lipidomics; Skin.
© 2025 The Authors.
Figures



Similar articles
-
Impaired epidermal ceramide synthesis causes autosomal recessive congenital ichthyosis and reveals the importance of ceramide acyl chain length.J Invest Dermatol. 2013 Sep;133(9):2202-11. doi: 10.1038/jid.2013.153. Epub 2013 Apr 2. J Invest Dermatol. 2013. PMID: 23549421
-
Altered expression of epidermal lipid bio-synthesis enzymes in atopic dermatitis skin is accompanied by changes in stratum corneum lipid composition.J Dermatol Sci. 2017 Oct;88(1):57-66. doi: 10.1016/j.jdermsci.2017.05.005. Epub 2017 May 18. J Dermatol Sci. 2017. PMID: 28571749
-
Lipid functions in skin: Differential effects of n-3 polyunsaturated fatty acids on cutaneous ceramides, in a human skin organ culture model.Biochim Biophys Acta Biomembr. 2017 Sep;1859(9 Pt B):1679-1689. doi: 10.1016/j.bbamem.2017.03.016. Epub 2017 Mar 21. Biochim Biophys Acta Biomembr. 2017. PMID: 28341437 Free PMC article.
-
Corneocyte lipid envelope (CLE), the key structure for skin barrier function and ichthyosis pathogenesis.J Dermatol Sci. 2017 Oct;88(1):3-9. doi: 10.1016/j.jdermsci.2017.06.002. Epub 2017 Jun 8. J Dermatol Sci. 2017. PMID: 28623042 Review.
-
Ceramide biosynthesis in keratinocyte and its role in skin function.Biochimie. 2009 Jun;91(6):784-90. doi: 10.1016/j.biochi.2009.04.001. Epub 2009 Apr 11. Biochimie. 2009. PMID: 19364519 Review.
References
-
- Akiyama M. Corneocyte lipid envelope (CLE), the key structure for skin barrier function and ichthyosis pathogenesis. J Dermatol Sci. 2017;88:3–9. - PubMed
-
- Baron U., Bujard H. Tet repressor-based system for regulated gene expression in eukaryotic cells: principles and advances. Methods Enzymol. 2000;327:401–421. - PubMed
-
- Behne M., Uchida Y., Seki T., de Montellano P.O., Elias P.M., Holleran W.M. Omega-hydroxyceramides are required for corneocyte lipid envelope (CLE) formation and normal epidermal permeability barrier function. J Invest Dermatol. 2000;114:185–192. - PubMed
-
- Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. - PubMed
-
- Bouwstra J., Pilgram G., Gooris G., Koerten H., Ponec M. New aspects of the skin barrier organization. Skin Pharmacol Appl Skin Physiol. 2001;14(Suppl 1):52–62. - PubMed
LinkOut - more resources
Full Text Sources

