Fenugreek inhibits cathepsin G activity and suppresses the progression of malignant phenotypes in MCF-7 cells
- PMID: 40524916
- PMCID: PMC12168360
- DOI: 10.1016/j.bbrep.2025.102021
Fenugreek inhibits cathepsin G activity and suppresses the progression of malignant phenotypes in MCF-7 cells
Abstract
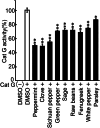
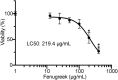
Spices and herbs, which are derived from natural botanical sources, contain many bioactive compounds and play an important role in human health. The general and specific health benefits of these spices and herbs include anti-inflammatory, antioxidant, and anti-tumorigenic activities. Previously, we showed that cathepsin G, which is a neutrophil-derived serine protease localized in human breast cancer tissues, promotes cancer metastasis via induction of platelet-activating factor acetylhydrolase 1B2 (PAFAH1B2) expression in MCF-7 human breast cancer cells. Therefore, although regulation of cathepsin G activity is thought to be important in human breast cancer progression, no compounds that inhibit the activity have been identified for therapeutic purposes. In this study, we screened 50 spice and herb extracts. Peppermint, clove, Sichuan pepper, and fenugreek exhibited strong inhibitory effects on cathepsin G activity and suppressed cathepsin G-induced MCF-7 cell aggregation.; importantly, fenugreek suppressed the increase in PAFAH1B2 expression. The IC50 of 37.38 μg/mL of fenugreek extract that showed inhibitory effect on cathepsin G-induced malignant progression was 5.87 times lower than the concentration that exerted cytotoxic effect. Interestingly, quercetin and trigonelline contained in fenugreek inhibited cathepsin G activity and suppressed the induction of cell aggregation and PAFAH1B2 expression in human breast cancer cells. These results suggest that quercetin and trigonelline are partly responsible for the inhibitory effect of fenugreek on cathepsin G-induced malignant progression of human breast cancer cells. Our findings provide a new breast cancer treatment strategy targeting cathepsin G, and fenugreek may have synergistic effects when combined with therapeutic drugs.
Keywords: Breast cancer; Cathepsin G; Fenugreek extracts; MCF-7; Platelet-activating factor acetylhydrorase 1B2; Quercetin; Trigonelline.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Cathepsin G-induced malignant progression of MCF-7 cells involves suppression of PAF signaling through induced expression of PAFAH1B2.Biochim Biophys Acta Mol Cell Biol Lipids. 2022 Aug;1867(8):159164. doi: 10.1016/j.bbalip.2022.159164. Epub 2022 Apr 22. Biochim Biophys Acta Mol Cell Biol Lipids. 2022. PMID: 35462067
-
Cytotoxicity of Fenugreek Sprout and Seed Extracts and Their Bioactive Constituents on MCF-7 Breast Cancer Cells.Nutrients. 2022 Feb 13;14(4):784. doi: 10.3390/nu14040784. Nutrients. 2022. PMID: 35215434 Free PMC article.
-
Neutrophil cathepsin G, but not elastase, induces aggregation of MCF-7 mammary carcinoma cells by a protease activity-dependent cell-oriented mechanism.Mediators Inflamm. 2014;2014:971409. doi: 10.1155/2014/971409. Epub 2014 Apr 2. Mediators Inflamm. 2014. PMID: 24803743 Free PMC article.
-
Herbs and Spices- Biomarkers of Intake Based on Human Intervention Studies - A Systematic Review.Genes Nutr. 2019 May 22;14:18. doi: 10.1186/s12263-019-0636-8. eCollection 2019. Genes Nutr. 2019. PMID: 31143299 Free PMC article. Review.
-
Health Benefits of Culinary Herbs and Spices.J AOAC Int. 2019 Mar 1;102(2):395-411. doi: 10.5740/jaoacint.18-0418. Epub 2019 Jan 16. J AOAC Int. 2019. PMID: 30651162 Review.
References
-
- Ginsburg O., Bray F., Coleman M.P., Vanderpuye V., Eniu A., Kotha S.R., Sarker M., Huong T.T., Allemani C., Dvaladze A., Gralow J., Yeates K., Taylor C., Oomman N., Krishnan S., Sullivan R., Kombe D., Blas M.M., Parham G., Kassami N., Conteh L. The global burden of women's cancers: a grand challenge in global health. Lancet. 2017;389:847–860. doi: 10.1016/S0140-6736(16)31392-7. - DOI - PMC - PubMed
-
- Tanigawa K., Kiriya M., Hayashi Y., Shinden Y., Kijima Y., Natsugoe S., Sumimoto T., Morimoto-Kamata R., Yui S., Hama K., Yokoyama K., Nakamura Y., Suzuki K., Nojiri H., Inoue K., Karasawa K. Cathepsin G-induced malignant progression of MCF-7 cells involves suppression of PAF signaling through induced expression of PAFAH1B2. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2022;1867 doi: 10.1016/j.bbalip.2022.159164. - DOI - PubMed
LinkOut - more resources
Full Text Sources

