Familial risk and phenotypic variation of sarcoidosis in the Icelandic population
- PMID: 40524927
- PMCID: PMC12168177
- DOI: 10.1183/23120541.00964-2024
Familial risk and phenotypic variation of sarcoidosis in the Icelandic population
Abstract
Background: The familial risk of sarcoidosis is heterogeneous, and previous studies demonstrate conflicting results. The present study maps the pattern of familial distribution of all known, biopsy-verified sarcoidosis in the Icelandic population (1981-2021).
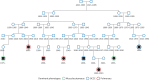
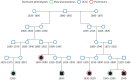
Methods: All cases were re-confirmed and categorised into one of the five phenotypic groups described by Schupp et al., and in different groups based on disease severity. Distant relationships were accurately traced for relatives of sarcoidosis patients and their mates using the nationwide Icelandic Genealogy Database. This allows creation of matched control groups for the calculation of relative risk and kinship coefficient (KC).
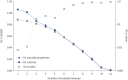
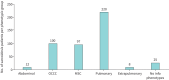
Results: 462 patients with biopsy-proven diagnoses of sarcoidosis were included. We identified 282 extended families and seven sibling pairs with sarcoidosis. 20 families had five or more affected individuals. Relative risk (RR) was 3.7 (95% CI 1.54-8.55) in 1st-degree relatives (p=0.003), 1.65 (1.05-1.92) in 4th-degree relatives (p=0.014) and 1.57 (1.08-1.70) in 5th-degree relatives (p=0.003). RRs among 1st-5th degree relatives of the patients' mates were not significant. KC for sarcoidosis was only significant for the first two meioses (KC=1.06, p<0.008 and KC=1.01, p=0.011, respectively). The most common sarcoidosis phenotypes were pulmonary-lymphonodal (47.6%), ocular-cardiac-cutaneous and central nervous system (21.6%), and musculoskeletal cutaneous (20.9%). There seems to be no clustering of a single phenotype or resistant sarcoidosis in the extended sarcoidosis families.
Conclusion: Our study does not consider heritability a strong risk factor for sarcoidosis. The risk is highest for 1st-degree relatives of patients with sarcoidosis. Single phenotypes and resistant sarcoidosis do not cluster in distinct families.
Copyright ©The authors 2025.
Conflict of interest statement
Conflict of interest: The authors of the manuscript declare no conflict of interest.
Figures
Similar articles
-
Familial aggregation of atrial fibrillation in Iceland.Eur Heart J. 2006 Mar;27(6):708-12. doi: 10.1093/eurheartj/ehi727. Epub 2006 Jan 20. Eur Heart J. 2006. PMID: 16428254
-
A strong heritability of psoriatic arthritis over four generations--the Reykjavik Psoriatic Arthritis Study.Rheumatology (Oxford). 2009 Nov;48(11):1424-8. doi: 10.1093/rheumatology/kep243. Epub 2009 Sep 9. Rheumatology (Oxford). 2009. PMID: 19741010
-
Prevalence and inheritance of hip osteoarthritis in Iceland.Acta Orthop Scand Suppl. 2000 Dec;298:1-46. Acta Orthop Scand Suppl. 2000. PMID: 11338422 Review.
-
Using the Icelandic genealogical database to define the familial risk of primary biliary cholangitis.Hepatology. 2018 Jul;68(1):166-171. doi: 10.1002/hep.29675. Epub 2018 May 6. Hepatology. 2018. PMID: 29159924
-
Clinical epidemiology of familial sarcoidosis: A systematic literature review.Respir Med. 2019 Mar;149:36-41. doi: 10.1016/j.rmed.2018.11.022. Epub 2018 Dec 13. Respir Med. 2019. PMID: 30587386
References
-
- Pietinalho A, Hiraga Y, Hosoda Y, et al. The frequency of sarcoidosis in Finland and Hokkaido, Japan. A comparative epidemiological study. Sarcoidosis 1995; 12: 61–67. - PubMed
LinkOut - more resources
Full Text Sources
