Biomarker profile and disease burden associated with intermittent and long-term oral corticosteroid use in patients with severe asthma prior to biologic initiation in real-life (STAR)
- PMID: 40524942
- PMCID: PMC12169735
- DOI: 10.1016/j.waojou.2025.101066
Biomarker profile and disease burden associated with intermittent and long-term oral corticosteroid use in patients with severe asthma prior to biologic initiation in real-life (STAR)
Abstract
Background: Asthma characterization using blood eosinophil count (BEC) (among other biomarkers and clinical indices) is recommended in severe asthma (SA), but the masking effect of oral corticosteroids (OCS), makes this challenging.
Aim: Our aim was to explore the effect of OCS use (both intermittent [iOCS] and long-term [LTOCS]) prior to biologic initiation on SA phenotype and biomarker profile in real-life and to characterize the burden of SA among patients prescribed LTOCS by biomarker profile.
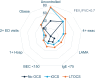
Methods: This was a registry-based cohort study, including data from 23 countries collected between 2003 and 2023 and shared with the Internatonal Severe Asthma Registry (ISAR). Patients with SA were categorized into 3 cohorts, those with: (i) no prescription for OCS, (ii) prescription(s) for iOCS (ie, ≤90 days in previous 12-months, usually short courses for exacerbations), and (iii) prescriptions for LTOCS (ie, >90 days in previous 12-months). Biomarker distribution (ie, BEC, fractional exhaled nitric oxide [FeNO], and total Immunoglobulin E [IgE]) were quantified in the year prior to biologic initiation in patients with SA according to OCS prescription pattern. Phenotypes were characterized for those prescribed LTOCS according to BEC cut-off (<150 and ≥ 150 cells/μL).
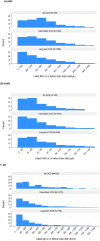
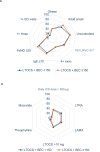
Results: Of 4305 patients included, 5.0% (n = 215), 54.1% (n = 2330) and 40.9% (n = 1760) were prescribed no OCS, iOCS, and LTOCS, respectively. The BEC distribution varied by prescription pattern and LTOCS dose (<5 mg to ≥20 mg/day); BEC was <150 cells/μL in 28.6% (n = 369/1288) of LTOCS patients, compared to 19.5% (n = 284/1460) of iOCS patients and 14.0% (n = 21/150) of those in the no OCS group. Median BEC was also significantly lower in the LTOCS versus the iOCS group (310 vs 400 cells/μL; p < 0.001). A similar pattern was noted for IgE, but not FeNO. Among LTOCS patients with BEC <150 cells/μL, 39.9% experienced ≥4 exacerbations, 75.1% had uncontrolled asthma symptoms and 55.9% had evidence of persistent airflow obstruction (compared with 40.9%, 76.2% and 59.5% of those with BEC ≥150 cells/μL, respectively).
Conclusions: OCS, whether prescribed intermittently or long term, affect BEC distribution potentially leading to heightened risk of phenotype misclassification and influencing subsequent treatment decisions. FeNO appears to be less susceptible to OCS-induced suppression. Disease burden was high for those in the LTOCS group and was high independent of dose and BEC. Our findings highlight the importance of considering OCS use, even intermittent use, when characterizing SA, and suggests the need for earlier phenotyping and alternative treatment strategies for LTOCS patients with low BEC.
Keywords: BEC; Blood eosinophil count; FeNO; Fractional exhaled nitric oxide; IgE; Immunoglobulin E; Intermittent; Long-term.
© 2025 The Author(s).
Conflict of interest statement
Florence Schleich reports consultancy work for GlaxoSmithKline, AstraZeneca, Sanofi - Advisory board, received speaker fees from GlaxoSmithKline, AstraZeneca, Chiesi, Teva, ALK and research grants from GlaxoSmithKline, AstraZeneca, and Chiesi. Désirée Larenas-Linnemann reports personal fees from ALK-Abelló, AstraZeneca national and global, Bayer, Chiesi, Grunenthal, Grin, GlaxoSmithKline national and global, Viatris, Novartis, Pfizer, Sanofi, Siegfried, and Carnot, grants for guideline development from Abbvie, Bayer, Chiesi, Lilly, Sanofi, AstraZeneca, Pfizer, Novartis, Circassia, UCB, and GlaxoSmithKline, outside the submitted work. Alan Altraja has received lecture fees from AstraZeneca, Berlin-Chemie Menarini, Boehringer Ingelheim, CSL Behring, GlaxoSmithKline, Merck, Sharp & Dohme, Norameda, Novartis, Orion, Sanofi, Takeda, Teva, and Zentiva; sponsorships from AstraZeneca, Berlin-Chemie Menarini, Boehringer Ingelheim, CSL Behring, GlaxoSmithKline, Merck, Sharp & Dohme, Norameda, Novartis, Takeda, Teva, and Sanofi; and has participated in advisory boards for AstraZeneca, Boehringer Ingelheim, CSL Behring, GlaxoSmithKline, Merck, Sharp & Dohme, Novartis, Sanofi, Shire Pharmaceuticals, and Teva. Luis PérezdeLlano reports grants, personal fees, and non-financial support from AstraZeneca, personal fees and non-financial support from GlaxoSmithKline, grants, personal fees and non-financial support from Teva, personal fees and non-financial support from Chiesi, grants, personal fees and non-financial support from Sanofi, personal fees from Merck Sharp & Dohme, personal fees from Techdow Pharma, grants, personal fees and non-financial support from Faes Farma, personal fees from Leo-Pharma, grants and personal fees from Gebro, personal fees from Gilead, outside the submitted work. Konstantinos Kostikas received honoraria for presentations and consultancy fees from AstraZeneca, Boehringer Ingelheim, Chiesi, ELPEN, GlaxoSmithKline, Guidotti, Menarini, Pfizer, Sanofi, Specialty Therapeutics. He was an employee of AstraZeneca from 2 September to 29 November2024. Mohsen Sadatsafavi has received honoraria from AstraZeneca, Boehringer Ingelheim, Teva, and GlaxoSmithKline for purposes unrelated to the content of this manuscript and has received research funding from AstraZeneca and Boehringer Ingelheim directly into his research account from AstraZeneca for unrelated projects. Arnaud Bourdin has received industry-sponsored grants from AstraZeneca/MedImmune, Boehringer Ingelheim, Cephalon/Teva, GlaxoSmithKline, Novartis, Sanofi-Regeneron, and consultancies with AstraZeneca/MedImmune, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Regeneron-Sanofi, Med-in-Cell, Actelion, Merck, Roche, and Chiesi. Roy Alton Pleasants is a consultant for AstraZeneca and Grifols and receives research support through institutions from AstraZeneca and GlaxoSmithKline. Mark Hew declares grants and other advisory board fees (made to his institutional employer) from AstraZeneca, GlaxoSmithKline, Novartis, Sanofi, Teva, and Seqirus, for unrelated projects. Wenjia Chen reports no conflict of interest. Libardo Jiménez-Maldonado has received fees as advisory board participant and/or speaker from AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Novartis, and Sanofi-Aventis; has participated in clinical trials for AstraZeneca, Novartis and GlaxoSmithKline. Simon Couillard reports the following: he has received non-restricted research grants from the NIHR Oxford BRC, the Quebec Respiratory Health Research Network, the Fondation Québécoise en Santé Respiratoire, AstraZeneca, bioMérieux, and Sanofi-Genyme-Regeneron; he is the holder of the Association Pulmonaire du Québec's Research Chair in Respiratory medicine and is a Clinical research scholar of the Fonds de recherche du Québec; he received speaker honoraria from AstraZeneca, GlaxoSmithKline, Sanofi-Regeneron, and Valeo Pharma; he received consultancy fees for FirstThought, AstraZeneca, GlaxoSmithKline, Sanofi-Regeneron, Access Biotechnology and Access Industries; he has received sponsorship to attend/speak at international scientific meetings by/for AstraZeneca and Sanofi-Regeneron. He is an advisory board member and will have stock options for Biometry Inc – a company which is developing a FeNO device (myBiometry). He advised the Institut national d'excellence en santé et services sociaux (INESSS) for an update of the asthma general practice information booklet for general practitioners. Charlotte Suppli Ulrik reports personal fees for talks, participation in advisory boards etc. from AstraZeneca, GlaxoSmithKline, Teva, Boehringer Ingelheim, Orion Pharma, Sanofi Genzyme, TFF Pharmaceuticals, Covis Pharma, Berlin-Chemie, Takeda, Chiesi, Pfizer, Hikma Pharmaceuticals, and Novo Nordisk, outside the submitted work. Adeeb A. Bulkhi has received speaker lecture fees from AstraZeneca, GlaxoSmithKline, Sanofi, Novartis, Takeda, and ALK. He also participated in advisory boards with GlaxoSmithKline, Sanofi, and Novartis. Ming-Ju Tsai has received sponsorship to attend or speak at conferences, honoraria for lecturing or attending advisory boards, and research grants from the following companies: AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Pfizer, Shionogi and Orient EuroPharma. George C. Christoff declares relevant research support from AstraZeneca and Sanofi. Nikolaos G. Papadopoulos has been a speaker and/or advisory board member for Abbott, Abbvie, ALK, Asit Biotech, AstraZeneca, Biomay, Boehringer Ingelheim, GlaxoSmithKline, HAL, Faes Farma, Medscape, Menarini, Merck Sharp & Dohme, Novartis, Nutricia, OM Pharma, Regeneron, Sanofi, Takeda, and Viatris. Paul E. Pfeffer has attended advisory boards for AstraZeneca, GlaxoSmithKline, and Sanofi; has given lectures/webinars at meetings supported by AstraZeneca, Chiesi, and GlaxoSmithKline; has taken part in clinical trials sponsored by AstraZeneca, GlaxoSmithKline, Novartis, Regeneron, and Sanofi, for which his institution received remuneration; and has current research grants funded by GlaxoSmithKline and a quality improvement grant funded by AstraZeneca. Dermot Ryan has (in the last 3 years) lectured on behalf of, received sponsorship from, or acted as a paid advisor to Mylan, AZ, Chiesi, Novartis, GlaxoSmithKline, Boehringer Ingelheim and Regeneron. Celine Bergeron reports advisory board participation of Sanofi-Regeneron, AstraZeneca, Takeda, ValeoPharma, consultant for Areteia, honorarium for presentations for AstraZeneca/Amgen, GlaxoSmithKline, Grifols, Sanofi-Regeneron, ValeoPharma and grants paid to The University of British Columbia from BioHaven, Sanofi-Regeneron, AstraZeneca, and GlaxoSmithKline. Mona S. Al-Ahmad has received advisory board and speaker fees from AstraZeneca, Sanofi, Novartis, and GlaxoSmithKline, and received a grant from Kuwait Foundation for the Advancement of Sciences (KFAS). Delbert R. Dorscheid is on faculty at the University of British Columbia and is supported by the following grants: Canadian Institutes of Health Research, British Columbia Lung Association, and Michael Smith Foundation for Health Research. In addition, he has received speaking fees, travel grants, unrestricted project grants, writing fees and is a paid consultant for Pharma including Sanofi Regeneron, Novartis Canada, AstraZeneca, GlaxoSmithKline and ValeoPharma. He is an active member of Canadian Thoracic Society, American Thoracic Society, European Respiratory Society, and the Allergen Research Network. Dr. Dorscheid does not believe that any of the disclosed potential conflicts represent true conflicts with respect to the information and recommendations included in this manuscript. Eileen Wang has received honoraria from AstraZeneca, GlaxoSmithKline, Amgen, and Genentech. She has been an investigator on studies sponsored by AstraZeneca, GlaxoSmithKline, Genentech, and Sanofi, for which her institution has received funding. John D. Blakey has received grants or contracts from Asthma Australia, MRFF, FHRI, Telethon Kids Institute, International Primary Care Respiratory Group, and the Charlies Foundation for Research. He has received payment or honoraria for lectures, presentations, or speaker bureaus from Chiesi, The Limbic, Boehringer Ingelheim, GlaxoSmithKline, AstraZeneca, and Sanofi. He has received support for attending meetings and/or travel from GlaxoSmithKline, Centre for Research Excellence in Treatable Traits, AstraZeneca, and The George Institute. He has participated in the study steering committee of GlaxoSmithKline, unrelated to this work. He has taken a leadership role in the Thoracic Society of Australia and New Zealand, and Asthma Australia. He has received medical writing/equipment from GlaxoSmithKline, and Novartis. Belinda Cochrane has received advisory fees from GlaxoSmithKline and Sanofi, speaker fees from AstraZeneca, Moderna and Chiesi, and research grant funding from GlaxoSmithKline. Matthew J. Peters declares personal fees and non-financial support from AstraZeneca, GlaxoSmithKline, and Sanofi. Todor A. Popov declares research support from Novartis and Chiesi Pharma, not related to this particular work. Carlos A. Torres-Duque has received fees as advisory board participant and/or speaker from AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Novartis, and Sanofi-Aventis; has taken part in clinical trials from AstraZeneca, Novartis and Sanofi-Aventis; has received unrestricted grants for investigator-initiated studies at Fundacion Neumologica Colombiana from AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Grifols, and Novartis. Susanne Hansen declares no relevant conflict of interest. Francesca Puggioni reports having received lectures or advisory board fees from Menarini, Mundipharma, Chiesi, Alk Abello, AstraZeneca, Boehringer Ingelheim, Guidotti, Malesci, GlaxoSmithKline, Hal Allergy, Novartis, Sanofi, Regeneron, Stallergenes Greer, Valeas, and Almirall. Kirsty Fletton is an employee of Optimum Patient Care Global (OPCG), a co-funder of the International Severe Asthma Registry. Laila Salameh declares no relevant conflict of interest. Peter G. Middleton declares grant support from HCF Australia, Canadian Institutes of Health, Board membership of the CF Foundation DSMB, and lecture fees and advisory boards for AstraZeneca, Limbic, MedEd, and Vertex, all unrelated to this work. Paulo Márcio Pitrez received fees as a speaker or for consultations from GlaxoSmithKline, AstraZeneca, Sanofi, and Aché. Chin Kook Rhee received consulting/lecture fees from Merck Sharp & Dohme, AstraZeneca, GlaxoSmithKline, Novartis, Takeda, Mundipharma, Boehringer-Ingelheim, Teva, Sanofi, Organon, Roche, and Bayer. Eve Denton declares grants to her institution from AstraZeneca, GlaxoSmithKline, Novartis, Sanofi, Teva, and Seqirus, for unrelated projects and speaker fees from Sanofi, and Boehringer Ingelheim. Kenneth R. Chapman has received grants from AstraZeneca, Boehringer Ingelheim, Bellus, CSL Behring, GlaxoSmithKline, Grifols, Inhibrx, Novartis, Regeneron, Sanofi, Takeda, Vertex, consulting fees from AstraZeneca, CSL Behring, GlaxoSmithKline, Grifols, Inhibrx, Novartis, Sanofi, Takeda. He has a leadership or fiduciary role in Alpha-1 Canada, Canadian Thoracic Society, Alpha-1 Foundation, and AlphaNet Canada. Lauri Lehtimäki has received personal fees from ALK, AstraZeneca, Berlin Chemie, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Menarini, Novartis, Orion Pharma and Sanofi. Ruth B. Murray is a consultant for Observational and Pragmatic Research Institute (OPRI) which conducted this study in collaboration with Optimum Patient Care, a co-funder of the International Severe Asthma Registry. Chau-Chyun Sheu has received advisory board and speaker fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, and Pfizer, and has acted as an investigator for trials sponsored by AstraZeneca, Novartis, Roche, Sanofi-Regeneron, Galapagos, Shionogi, Aridis, Bristol Myers Squibb, Insmed, United Therapeutics, Enanta Pharmaceuticals, Areteia Therapeutics, Meiji, and Horizon Therapeutics. David J. Jackson has received speaker fees and consultancy fees from AstraZeneca, GlaxoSmithKline, Sanofi Regeneron, and Boehringer Ingelheim, and research funding from AstraZeneca. Riyad Al-Lehebi has given lectures at meetings supported by AstraZeneca, Boehringer Ingelheim, Novartis, GlaxoSmithKline, and Sanofi, and participated in advisory board fees from GlaxoSmithKline, AstraZeneca, Novartis, and Abbott. Mariko Siyue Koh reports grant support from AstraZeneca, and honoraria for lectures and advisory board meetings paid to her hospital (Singapore General Hospital) from GlaxoSmithKline, AstraZeneca, Novartis, Sanofi, and Boehringer Ingelheim, outside the submitted work. Bassam Mahboub reports no conflict of interest. Ledit R. F. Adrusso is an investigator and/or speaker for AstraZeneca, GlaxoSmithKline, Novartis, Sanofi, Amgen, Chiesi, Bellus, Areteria, Insmed, and Optinose. Athena Gogali received honoraria for presentations and consultancy fees from: AstraZeneca, Boehringer Ingelheim, Chiesi, ELPEN, GlaxoSmithKline, Novartis, and Menarini. Giorgio Walter Canonica has received research grants, as well as lecture or advisory board fees from A. Menarini, Alk-Albello, Allergy Therapeutics, Anallergo, AstraZeneca, MedImmune, Boehringer Ingelheim, Chiesi Farmaceutici, Circassia, Danone, Faes, Genentech, Guidotti Malesci, GlaxoSmithKline, Hal Allergy, Merck, Merck Sharp & Dohme, Mundipharma, Novartis, Orion, Sanofi Aventis, Sanofi, Genzyme/Regeneron, Stallergenes, UCB Pharma, Uriach Pharma, Teva, Thermo Fisher, and Valeas. Piotr Kuna reports personal fees from Adamed, AstraZeneca, Berlin Chemie Menarini, FAES, Glenmark, Novartis, Polpharma, Boehringer Ingelheim, Teva, Zentiva, outside the submitted work. Martin Sivori has received lecture fees for medical education programs of AstraZeneca, GlaxoSmithKline, and TEVA. Renaud Louis has received grants and lecture fees from AstraZeneca, GlaxoSmithKline, and Sanofi. Shelley Abercromby declares no relevant conflict of interest. Giuseppe Guida received lecture fees from GlaxoSmithKline and AstraZeneca. Bernt Bøgvald Aarli reports grants from AstraZeneca and Novartis, honoraria for presentations from AstraZeneca, GlaxoSmithKline, Sanofi-Aventis, and Orion, consulting fees from AstraZeneca, Grifols, and IM Medical Education Nordic AB, and has participated in advisory boards for AstraZeneca, GlaxoSmithKline, Chiesi, Sanofi-Aventis, Grifols, Orion, and Celltrion Healthcare, and he reports owning stocks in KBB Medic (Medical app company), outside the submitted work. His institution has received funding from Novartis and AstraZeneca. Aaron Beastall is an employee of Optimum Patient Care Global, a co-funder of the International Severe Asthma Registry. Victoria Carter is an employee of Optimum Patient Care (OPC). OPC is a co-funder of the International Severe Asthma Registry. Ghislaine Scelo is a consultant for Observational and Pragmatic Research Institute (OPRI). OPRI conducted this study in collaboration with Optimum Patient Care, a co-funder of the International Severe Asthma Registry. John Townend is an employee of the Observational and Pragmatic Research Institute (OPRI). OPRI conducted this study in collaboration with Optimum Patient Care, a co-funder of the International Severe Asthma Registry. Borja G. Cosio declares grants from Chiesi, Menarini, and GlaxoSmithKline; personal fees for advisory board activities from Chiesi, GlaxoSmithKline, Novartis, Sanofi, Teva, and AstraZeneca; and payment for lectures/speaking engagements from Chiesi, Novartis, GlaxoSmithKline, Menarini, and AstraZeneca, outside the submitted work. Pujan H. Patel has received advisory board and speaker fees from AstraZeneca, GlaxoSmithKline, Novartis, and Sanofi/Regeneron. Celine Yun Yi Goh is an employee of Optimum Patient Care Global (OPCG), a co-funder of the International Severe Asthma Registry. Zsuzsanna Csoma declares no relevant conflict of interest. John W. Upham has received speaker and consulting fees from Novartis, AstraZeneca, GlaxoSmithKline, Sanofi, and Boehringer Ingelheim. João A. Fonseca reports grants from research agreements with AstraZeneca, Mundipharma, Sanofi Regeneron, and Novartis. Personal fees for lectures and attending advisory boards: AstraZeneca, GlaxoSmithKline, Mundipharma, Novartis, Sanofi Regeneron, and Teva. Peter G. Gibson has received speaker fees and grants to his institution from AstraZeneca, GlaxoSmithKline, and Novartis. Christine Jenkins is a member of advisory boards for AstraZeneca, GlaxoSmithKline and Chiesi. She has received payment for Advisory board attendance, honoraria, lectures and meeting participation as a speaker. She has no conflicts in relation to this work. Guy G. Brusselle has received honoraria for lectures from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, and Novartis. He is a member of advisory boards for AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Merck Sharp & Dohme (MSD), Novartis, and Sanofi/Regeneron. Anne Chèvremont declares no conflict of interest. Andréanne Côté declares she has received speaking fees and consultant fees from Sanofi, Regeneron, AstraZeneca, and GlaxoSmithKline. She received unrestricted grant support from GlaxoSmithKline, and AstraZeneca. Carlos Andrés Celis-Preciado declares no relevant conflict of interest. Ivan Solarte has received fees as an advisory board participant and/or speaker from GlaxoSmithKline, AstraZenca, and Sanofi. Celeste M. Porsbjerg has attended advisory boards for AstraZeneca, Novartis, TEVA, and Sanofi-Genzyme; has given lectures at meetings supported by AstraZeneca, Novartis, TEVA, Sanofi-Genzyme, and GlaxoSmithKline; has taken part in clinical trials sponsored by AstraZeneca, Novartis, Merck Sharp & Dohme, Sanofi-Genzyme, GlaxoSmithKline, and Novartis; and has received educational and research grants from AstraZeneca, Novartis, TEVA, GlaxoSmithKline, ALK, and Sanofi-Genzyme. Asger Sverrild has attended advisory boards for Sanofi, and GSK, has given lectures at meetings supported by AstraZeneca, GSK, and Sanofi, and has taken part in clinical trials sponsored by AstraZeneca, Sanofi, and Eli Lilly. Paula Kauppi has received lecture fees from GlaxoSmithKline, consultancy fees from Sobi, and PI fees from Theravance. Stelios Loukides has received fees and honoraria from Menarini, GlaxoSmithKline, Novartis, Elpen, Pfizer, Gilead, Guidotti, AstraZeneca, and Chiesi. Michael P. Makris reports honoraria for presentations and consultancy fees from Novartis, GlaxoSmithKline, Menarini, AstraZeneca, Chiesi, Sanofi, Pfizer, outside the submitted work. Andriana I. Papaioannou has received fees and honoraria from Menarini, GlaxoSmithKline, Novartis, Elpen, Boehringer Ingelheim, AstraZeneca, Demo, and Chiesi. Enrico Heffler declares personal fees for advisory boards participation and/or speaker activities from: Sanofi, Regeneron, GlaxoSmithKline, Novartis, AstraZeneca, Stallergenes-Greer, Circassia, Bosch, Celltrion-Healthcare, Chiesi, and Almirall. Jeffrey Shi Kai Chan is an employee of the Observational and Pragmatic Research Institute (OPRI). OPRI conducted this study in collaboration with Optimum Patient Care, a co-funder of the International Severe Asthma Registry. Hyonsoo Joo reports no conflict of interest. Liam G. Heaney has received grant funding, participated in advisory boards and given lectures at meetings supported by Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Hoffmann la Roche, GlaxoSmithKline, Novartis, Theravance, Evelo Biosciences, Sanofi, and Teva; he has received grants from MedImmune, Novartis UK, Roche/Genentech Inc, GlaxoSmithKline, Amgen, Genentech/Hoffman la Roche, AstraZeneca, MedImmune, Aerocrine, and Vitalograph; he has received sponsorship for attending international scientific meetings from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, and Napp Pharmaceuticals; he has also taken part in asthma clinical trials sponsored by AstraZeneca, Boehringer Ingelheim, Hoffmann la Roche, and GlaxoSmithKline for which his institution received remuneration; he is the Academic Lead for the Medical Research Council Stratified Medicine UK Consortium in Severe Asthma which involves industrial partnerships with a number of pharmaceutical companies including Amgen, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Hoffmann la Roche, and Janssen. Wei-Han Cheng was an employee of Sanofi and may hold stock and/or stock options in the company. Njira Lugogo received consulting fees from Amgen, AstraZeneca, Avillion, Genentech, GSK, Niox, Novartis, Regeneron, Sanofi, and Teva; honoraria for non-speakers bureau presentations from GSK, TEVA and Astra Zeneca; and travel support from Astra Zeneca, SANOFI, TEVA, Regeneron and GSK; her institution received research support from Amgen, AstraZeneca, Avillion, Bellus, Evidera, Gossamer Bio, Genentech, GSK, Janssen, Niox, Regeneron, Sanofi, Novartis and Teva. She is an honorary faculty member of Observational and Pragmatic Research Institute (OPRI) but does not receive compensation for this role. Michael E. Wechsler reports grants and/or personal fees from Novartis, Sanofi, Regeneron, Genentech, Sentien, Restorbio, Equillium, Genzyme, Cohero Health, Teva, Boehringer Ingelheim, AstraZeneca, Amgen, GlaxoSmithKline, Cytoreason, Cerecor, Sound Biologics, Incyte, and Kinaset. Cláudia Chaves Loureiro has received (in the last 3 years) lecture or advisory board fees from AstraZeneca, GlaxoSmithKline, Menarini, Sanofi, and Teva, outside this work. Bellanid Rodríguez-Cáceres declares no relevant conflict of interest. Tatsuya Nagano received lecture fees from Kyorin, Sanofi, GlaxoSmithKline, Novartis, and AstraZeneca. Zhixiao Wang is an employee of Regeneron Pharmaceuticals, Inc, and holds stock and/or stock options in the company. Hao-Chien Wang reports no conflict of interest. Jorge Máspero reports speaker fees, grants, or advisory boards for AstraZeneca, Sanofi, GlaxoSmithKline, Novartis, Inmunotek, Menarini, and Noucor. Fernando Saldarini is a speaker of GlaxoSmithKline, AstraZeneca, and SANOFI. Ana María Stok has acted as an investigator for GlaxoSmithKline, AstraZeneca, Sanofi, Chiesi, Novartis, and Bago. She reports speaker fees from GlaxoSmithKline, AstraZeneca, and Sanofi. Anahi Yañez has received grants/research supports from GlaxoSmithKline, AstraZeneca, Sanofi, Chiessi, Novartis, MDS, Roche, Faes, TEVA, Avillon, Janssen, Bayer, Sanofi Gynzene, and he has received consultation fees from GlaxoSmithKline, AstraZeneca, Sanofi, and Eurofarma. He has participated in a company sponsored speaker's bureau from Sanofi, GlaxoSmithKline, Faes, Sanofi Gynzene, and AstraZeneca. Philip G. Bardin declares no relevant conflict of interest. Sinthia Z. Bosnic-Anticevich has received honorarium for participation in expert advisory boards and given lectures for Teva Pharmaceuticals, AstraZeneca, GlaxoSmithKline, Meda/Mylan, Sanofi, Mylan, Chiesi, Menarini, Sanofi, Boehringer Ingelheim, Abbvie and received unrestricted research grants from Mylan, AstraZeneca, Teva, AstraZeneca, GlaxoSmithKline, and Viatris. Vidya Navaratnam was an employee of OPCA when she worked on this manuscript, but she has left OPCA. She has received payment for lectures/speaking engagements from Boehringer Ingelheim as well as payment for travel/accommodation/meeting expenses from Boehringer Ingelheim and Bristol Myer Squibs. Mohit Bhutani has received speaker and consultant fees for AstraZeneca, GlaxoSmithKline, Sanofi, Covis, Boerhinger Ingelheim, Takeda and Valeo. He has leadership roles within the Canadian Thoracic Society. M. Diane Lougheed has (in the past 3 years) received grants outside the submitted work paid directly to Queen's University from the Canadian Institutes of Health Research (sub-grant from Ottawa Health Research Institute), Manitoba Workers Compensation Board, Ontario Lung Association, Ontario Thoracic Society, the Government of Ontario's Innovation Fund, Queen's University, Astra Zeneca and GlaxoSmithKline; and honoraria from the Canadian Thoracic Society for co-development and co-presentation of as Severe Asthma PREP course, from MD Breifcase for co-development of an accredited CME module on Severe Asthma; and from AstraZeneca for participation in the Precision Program Advisory Board. She has also served as a member and past-chair of the Canadian Thoracic Society (CTS) Asthma Clinical Assembly, member of the CTS Asthma Clinical Assembly Steering Committee, CTS representative on the Lung Association's Board of Directors, CTS representative to the European Respiratory Society, and member of Health Quality Ontario's Asthma in Adults and Asthma in Children Quality Standard Advisory Committee. Lyle Melenka † declares no conflict of interest. Petros Bakakos declares no conflict of interest. Konstantinos P. Exarchos declares no relevant conflict of interest. Aggelos A. Ladias declares no conflict of interest. Dóra Lúdvíksdóttir has received lecture fees from GlaxoSmithKline, Sanofi and AstraZeneca. Takashi Iwanaga received speaker bureau fees from Kyorin, GlaxoSmithKline, Novartis, Boehringer Ingelheim, AstraZeneca, and Sanofi. Elvia Angelica Contreras Contreras declares no relevant conflict of Interest. Sverre Lehmann has been an investigator on clinical trials sponsored by GlaxoSmithKline and AstraZeneca, for which his institution has received funding. José Alberto Ferreira declares no conflict of interest. Rebecca Gall is an employee and shareholder of Regeneron Pharmaceuticals, Inc. Pin-Kuei Fu declares no relevant conflict of interest. Diahn-Warng Perng received sponsorship to attend or speak at international meetings, honoraria for lecturing or attending advisory boards, and research grants from the following companies: AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Daiichi Sankyo, Shionogi, and Orient Pharma. Flavia Hoyte declares honoraria from AstraZeneca and Genentech. She has been an investigator on clinical trials sponsored by GlaxoSmithKline, Genentech, Teva, Sanofi, for which her institution has received funding. Rohit Katial declares no relevant conflict of interest. Unnur S. Björnsdóttir receives gratuities for lectures/presentations from AstraZeneca, Sanofi and Novartis. Camille Taillé has received lecture or advisory board fees and grants to her institution from AstraZeneca, Sanofi, GlaxoSmithKline, Chiesi, Stallergenes, Celltrion and Novartis, for unrelated projects. Christian Taube declares no relevant conflict of interest. Breda Cushen has received honoraria for lectures and received sponsorship for attending meetings from AstraZeneca, Novartis, and Boehringer Ingelheim. She has participated in advisory boards, and provided consultancy,for Chiesi and Sanofi. Lakmini Bulathsinhala is an employee of the Observational and Pragmatic Research Institute (OPRI). OPRI conducted this study in collaboration with Optimum Patient Care, a co-funder of the International Severe Asthma Registry. Leif Bjermer has (in the last 3 years) received lecture or advisory board fees from Alk-Abello, AstraZeneca, Chiesi, GlaxoSmithKline, Sanofi, and Genzyme/Regeneron. David B. Price has advisory board membership with AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Viatris, Teva Pharmaceuticals; consultancy agreements with AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Viatris, Teva Pharmaceuticals; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from AstraZeneca, Chiesi, Viatris, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, and UK National Health Service; payment for lectures/speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Inside Practice, GlaxoSmithKline, Medscape, Viatris, Novartis, Regeneron Pharmaceuticals and Sanofi Genzyme, Teva Pharmaceuticals; payment for travel/accommodation/meeting expenses from AstraZeneca, Boehringer Ingelheim, Novartis, Medscape, Teva Pharmaceuticals.; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 92.61% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); is peer reviewer for grant committees of the UK Efficacy and Mechanism Evaluation Programme, and Health Technology Assessment; and he was an expert witness for GlaxoSmithKline.
Figures
References
-
- Global Strategy for Asthma Management and Prevention . Global Initiative for Asthma; 2024. https://ginasthma.org/wp-content/uploads/2024/05/GINA-2024-Strategy-Repo... Published online.
LinkOut - more resources
Full Text Sources

