Melatonin Activates KEAP1/NRF2/PTGS2 Pathway to Attenuate Hyperoxia-Driven Ferroptosis in Bronchopulmonary Dysplasia
- PMID: 40524969
- PMCID: PMC12168915
- DOI: 10.2147/JIR.S520404
Melatonin Activates KEAP1/NRF2/PTGS2 Pathway to Attenuate Hyperoxia-Driven Ferroptosis in Bronchopulmonary Dysplasia
Abstract
Background and purposes: Ferroptosis, a type of regulated cell death, has been confirmed to play a role in the pathogenesis of bronchopulmonary dysplasia (BPD). This study aimed to test the hypothesis that melatonin mitigates hyperoxia-induced BPD by inhibiting ferroptosis in alveolar epithelial cells, specifically through modulation of the KEAP1/NRF2/PTGS2 signaling pathway.
Methods: Hyperoxia-induced MLE-12 cells and neonatal mice were used to establish BPD models. The effects of melatonin on hyperoxia-induced ferroptosis in MLE-12 cells were assessed by administering melatonin and ferroptosis inducer erastin to these cells. Key target genes involved in melatonin's ameliorative effects on BPD were identified using bioinformatics analysis. To confirm the regulatory relationship between melatonin and the KEAP1/NRF2/PTGS2 pathway, MLE-12 cells were treated with the NRF2 inhibitor ML385 under hyperoxic conditions. Additionally, molecular docking was performed to predict interactions between melatonin and KEAP1.
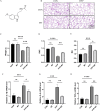
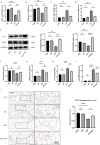
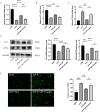
Results: Melatonin (MT) treatment up-regulated the expression of glutathione peroxidase 4 (GPX4) and xCT in hyperoxia-treated alveolar epithelial cells. The anti-ferroptosis effect of MT on these cells was significantly reduced by ML385, confirming the role of the KEAP1/NRF2 pathway in MT's mechanism of action. In vivo experiments demonstrated that MT up-regulated NRF2, GPX4, and xCT levels and down-regulated KEAP1 and PTGS2 levels in hyperoxia-induced BPD models.
Conclusion: Melatonin exerts a protective effect against hyperoxia-induced BPD by inhibiting ferroptosis in alveolar epithelial cells, and this effect is mediated, at least in part, through the KEAP1/NRF2/PTGS2 axis.
Keywords: KEAP1; NRF2; PTGS2; bronchopulmonary dysplasia; ferroptosis; melatonin.
© 2025 Deng et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials

