Triphenylphosphine-Based Mitochondrial Targeting Nanocarriers: Advancing Cancer Therapy
- PMID: 40524980
- PMCID: PMC12168994
- DOI: 10.2147/CPAA.S526895
Triphenylphosphine-Based Mitochondrial Targeting Nanocarriers: Advancing Cancer Therapy
Abstract
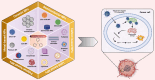
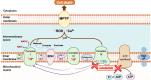
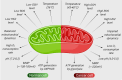
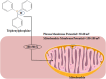
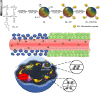
Numerous chemotherapeutic drugs are commercially available for cancer treatment; however, their efficacy is often compromised by diminishing therapeutic effectiveness and unpredictable adverse effects. The lack of specific targeting limits their optimal therapeutic potential. Mitochondria are the primary sites of cellular energy production and play a critical role in cell survival and death. Furthermore, numerous studies have found an apparent association between mitochondrial metabolism and carcinogenesis and progression. Therefore, significant attention has been directed toward nanocarriers specifically designed for mitochondrial delivery, aiming to enhance the precision of chemotherapeutic agent transport to these critical organelles. Among these, triphenylphosphonium has emerged as a prominent mitochondrial targeting agent due to its superior targeting capabilities. This approach not only reduces the required drug dosage but also minimizes adverse effects on healthy tissues. This review provides a concise analysis of nanotechnology's contributions to cancer therapy, emphasizing its potential for targeting at both cellular and sub-cellular levels. Additionally, it delves into mitochondrial targeting, with a particular focus on nanocarriers engineered for efficient mitochondrial drug delivery. Moreover, it focuses on strategies employed by researchers to introduce TPP in nanocarrier systems for mitochondrial delivery and concludes by addressing challenges associated with TPP including hemolytic activity and how researchers mitigate this issue.
Keywords: cancer; mitochondrial targeting; nanomedicine; nanoparticles; triphenylphosphine.
© 2025 Ali et al.
Conflict of interest statement
There is no conflict of interest and disclosures associated with the work.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources

