Establishing reference values for age-related fecal calprotectin in healthy children aged 0-4 years: a systematic review and meta-analysis
- PMID: 40525108
- PMCID: PMC12169166
- DOI: 10.7717/peerj.19572
Establishing reference values for age-related fecal calprotectin in healthy children aged 0-4 years: a systematic review and meta-analysis
Abstract
Background and aims: The use of fecal calprotectin (FC) as a biomarker in children under 4 years of age is limited, as widely accepted reference values have not yet been established. Thus, the present meta-analysis was performed to establish convincing age-related FC reference values.
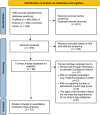
Method: We conducted a comprehensive meta-analysis to establish age-specific reference intervals for FC in children aged 0-4 years by synthesizing data from multiple studies. An exhaustive search of three major databases was conducted from their inception through April 2024, and this was complemented by manual screening of reference lists to ensure the inclusion of all relevant studies. The eligibility criteria were restricted to English-language publications reporting quantitative FC levels in apparently healthy children under 4 years of age. A stringent study selection protocol was applied, with each article independently reviewed by at least two investigators to ensure accuracy in study inclusion, data extraction, and methodological quality assessment. For the statistical analysis, random-effects meta-analysis models were constructed using restricted maximum likelihood estimation to generate pooled mean FC levels and corresponding 95% confidence intervals (CIs) at the study level.
Results: A total of 23 studies (n = 2,883) were included in the qualitative synthesis. The pooled mean FC reference values (μg/g) by age group were as follows: <1 month: 257.70 (95% CI [189.70-325.70]), 1-6 months: 239.46 (95% CI [181.17-297.75]), 7-12 months: 115.72 (95% CI [89.69-141.75]), 13-24 months: 104.70 (95% CI [61.96-147.44]), 25-36 months: 75.18 (95% CI [43.94-106.42]), and 37-48 months: 33.89 (95% CI [24.43-43.35]). Assay methodology, particularly enzyme-linked immunosorbent assay, demonstrated significant heterogeneity (p = 0.011) in manufacturer-specific analyses. Furthermore, geographical variation had a significant effect on baseline FC levels in neonates. In contrast, the type of feeding and mode of delivery did not show significant effects (p > 0.05).
Conclusion: We established normal reference ranges for FC in healthy children aged 0-4 years. Assay methodology and geographic factors significantly influence baseline FC levels, underscoring the need for careful interpretation of FC levels in clinical settings.
Keywords: Fecal calprotectin; Infant; Meta-analysis; Reference values.
© 2025 Zeng et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Brown MB, Wolfe RA. Estimation of the variance of percentile estimates. Computational Statistics & Data Analysis. 1983;1(1):167–174. doi: 10.1080/10543406.2019.1632872. - DOI
-
- Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database of Systematic Reviews. 2019;10(10):ED000142. doi: 10.1002/9781119536604. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

