Single-cell transcriptomics analysis of the healing process of ligament rupture
- PMID: 40525225
- PMCID: PMC12171888
- DOI: 10.1302/2046-3758.146.BJR-2024-0307.R3
Single-cell transcriptomics analysis of the healing process of ligament rupture
Abstract
Aims: The repair process of ligament ruptures is a complex phenomenon involving three stages: early, repair, and remodelling. This study aimed to investigate the cellular and genetic aspects related to the repair and healing of ligament ruptures using single-cell RNA sequencing (scRNA-seq) on anterior cruciate ligament (ACL) tissues.
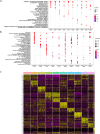
Methods: A comprehensive examination was conducted on ACL tissues from three healthy individuals and three patients with ligament ruptures at different timepoints (one week, three weeks, and six months post-injury). A deep gene expression analysis was performed on 83,195 cells obtained from the six cases, and immunohistochemistry techniques were used to identify cell types.
Results: In this study, tenocytes and fibroblasts in ligament tissues were distinctly identified for the first time. Moreover, a total of ten cell populations were discovered in ACL tissues, comprising tenocytes, fibroblasts, macrophages, stromal cells, T cells, endothelial cells, B cells, epithelial cells, chondrocytes, and monocytes. Further analysis of the tenocyte populations revealed ten distinct subtypes, highlighting the diversity of tenocytes in human ACL tissues.
Conclusion: The identification of multiple specialized tenocyte populations in human ACL tissues sheds light on potential avenues for advancing research in cell therapy for ligament injuries. These findings provide valuable insights into the cellular components involved in the repair and healing processes of ligament ruptures, paving the way for future investigations in this field.
© 2025 Zhao et al.
Conflict of interest statement
The authors report that this study was supported by grants from the Qingdao Shinan District Science and Technology Bureau public domain science and technology support plan project (2023-2-011-YY) and Shandong Province medical health science and technology project (202404070961).
Figures






Similar articles
-
Molecular feature-based classification of retroperitoneal liposarcoma: a prospective cohort study.Elife. 2025 May 23;14:RP100887. doi: 10.7554/eLife.100887. Elife. 2025. PMID: 40407808 Free PMC article.
-
Prediction, screening and characterization of novel bioactive tetrapeptide matrikines for skin rejuvenation.Br J Dermatol. 2024 Jun 20;191(1):92-106. doi: 10.1093/bjd/ljae061. Br J Dermatol. 2024. PMID: 38375775
-
Assessment of Return To Driving After Anterior Cruciate reconstruction.Orthop Traumatol Surg Res. 2025 May 31:104308. doi: 10.1016/j.otsr.2025.104308. Online ahead of print. Orthop Traumatol Surg Res. 2025. PMID: 40456389
-
Assessing the comparative effects of interventions in COPD: a tutorial on network meta-analysis for clinicians.Respir Res. 2024 Dec 21;25(1):438. doi: 10.1186/s12931-024-03056-x. Respir Res. 2024. PMID: 39709425 Free PMC article. Review.
-
The diagnostic accuracy of ultrasound in assessing anterior cruciate ligament tears: a systematic review and meta-analysis.Skeletal Radiol. 2025 Aug;54(8):1631-1642. doi: 10.1007/s00256-025-04866-w. Epub 2025 Jan 14. Skeletal Radiol. 2025. PMID: 39806120
References
Grants and funding
LinkOut - more resources
Full Text Sources

