Development of a Vinylated Cyclic Allene: A Fleeting Strained Diene for the Diels-Alder Reaction
- PMID: 40525234
- PMCID: PMC12338423
- DOI: 10.1002/anie.202510319
Development of a Vinylated Cyclic Allene: A Fleeting Strained Diene for the Diels-Alder Reaction
Abstract
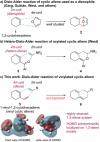
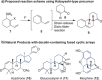
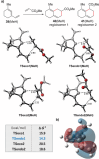
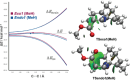
Fleeting molecules possessing strained multiple bonds are important components in organic synthesis due to their ability to undergo various chemical reactions driven by the release of strain energy. Although the use of strained π-bonds as 2π components, represented by dienophiles in Diels-Alder reactions, has been well studied, "the strained diene (4π component) approach" for molecular construction remains underexplored. Herein, we report the design of a vinyl cyclic allene (1-vinyl-1,2-cyclohexadiene) as a highly reactive strained diene and the development of its Diels-Alder reactions. Experimental and computational studies of vinyl cyclic allenes revealed that this diene system undergoes cycloaddition with dienophiles regio- and stereoselectively under mild reaction conditions. These studies also provide insight into the reactivity and selectivity of the system. The strained diene approach enables the convergent construction of polycyclic molecules through bond disconnections distinct from conventional retrosynthetic analysis, thus offering an efficient strategy for the assembly of functional molecules.
Keywords: Activation strain model; Carbocycles; Diels–Alder reaction; Strained diene; Vinylated cyclic allene.
© 2025 The Author(s). Angewandte Chemie International Edition published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Organic Synthesis Away from Equilibrium: Contrathermodynamic Transformations Enabled by Excited-State Electron Transfer.Acc Chem Res. 2024 Jul 2;57(13):1827-1838. doi: 10.1021/acs.accounts.4c00227. Epub 2024 Jun 21. Acc Chem Res. 2024. PMID: 38905487 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Light-Driven C(sp3)-C(sp3) Bond Functionalizations Enabled by the PCET Activation of Alcohol O-H Bonds.Acc Chem Res. 2025 Jul 1;58(13):2061-2071. doi: 10.1021/acs.accounts.5c00246. Epub 2025 Jun 13. Acc Chem Res. 2025. PMID: 40511721
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
References
-
- Liebman J. F., Greenberg A., Chem. Rev. 1976, 76, 311–365.
-
- Wiberg K. B., Angew. Chem. Int. Ed. 1986, 25, 312–322.
-
- Turkowska J., Durka J., Gryko D., Chem. Commun. 2020, 56, 5718–5734. - PubMed
-
- McDermott L., Walters Z. G., Clark A. M., Garg N. K., Nat. Synth. 2025, 4, 421–431.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

