A Double-Blind Randomised Clinical Trial of Terbinafine-Nanostructured Lipid Carriers: Should We Anticipate This Strategy for Effective Topical Treatment of Onychomycosis?
- PMID: 40525269
- PMCID: PMC12171949
- DOI: 10.1111/myc.70076
A Double-Blind Randomised Clinical Trial of Terbinafine-Nanostructured Lipid Carriers: Should We Anticipate This Strategy for Effective Topical Treatment of Onychomycosis?
Abstract
Background: Oral terbinafine (TBF) is the drug of choice for onychomycosis management. To treat and heal the rough and thick nail tissue affected by fungal agents, a high dose and plasma concentration of this drug is necessary. This, however, poses a life-threatening risk due to the cytotoxic side effects, drug-drug interactions, and adverse physical and chemical properties associated with oral medications.
Objectives: This study aimed to employ nanostructured lipid carriers (NLCs) in a gel formulation to avoid side effects and to increase the absorption of topical TBF.
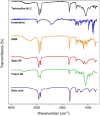
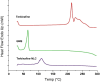
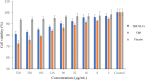
Methods: Terbinafine-loaded nanostructured lipid carriers (TBF-NLCs) were developed and optimised using an ultrasonic probe technique, resulting in the formulation of TBF-NLCs as a 1% w/w carbopol gel after verifying the characteristics associated with NLCs. In vitro antifungal susceptibility test (AFST) was conducted on 85 prevalent fungal species associated with onychomycosis, as well as on strains isolated from trial participants, following the CLSI M38-A2 and M27-A3 guidelines. A total of 60 volunteers were enrolled in this clinical randomised, double-blind, placebo-controlled study, divided equally into three groups prescribed with TBF cream 1%, TBF-NLCs gel 1%, and a placebo.
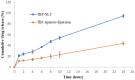
Results: A monodisperse suspension of spherical nanoparticles was successfully produced, exhibiting a zeta potential of 18.4 ± 1.02 mV, a Z-average of 131.7 ± 5.32 nm, a PDI index of 0.280 ± 0.017, and an EE percentage of 83.51 ± 3.52, all without any cytotoxic effects. The severity index showed a reduction from 65% and 55% to 35% and 10% in the TBF cream 1% and TBF-NLCs groups, respectively. From a mycological perspective, no significant negative results were noted during the 6th and 8th weeks of TBF-NLC 1% gel application.
Conclusion: The application of TBF-NLCs gel 1% demonstrated a quicker clinical recovery without adverse side effects compared to TBF cream, thus highlighting the effective nature of NLCs.
Keywords: NLCs; TBF‐NLC 1% gel; antifungal susceptibility test; clinical trial; mycological perspective; onychomycosis; severity index; terbinafine.
© 2025 The Author(s). Mycoses published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Oral antifungal medication for toenail onychomycosis.Cochrane Database Syst Rev. 2017 Jul 14;7(7):CD010031. doi: 10.1002/14651858.CD010031.pub2. Cochrane Database Syst Rev. 2017. PMID: 28707751 Free PMC article.
-
Betulin-NLC-hydrogel for the Treatment of Psoriasis-like Skin Inflammation: Optimization, Characterisation, and In vitro and In vivo Evaluation.Curr Drug Deliv. 2025;22(5):627-647. doi: 10.2174/0115672018329544240922151617. Curr Drug Deliv. 2025. PMID: 39360545
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Topical clonidine for neuropathic pain in adults.Cochrane Database Syst Rev. 2022 May 19;5(5):CD010967. doi: 10.1002/14651858.CD010967.pub3. Cochrane Database Syst Rev. 2022. PMID: 35587172 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
References
-
- Havlickova B., Czaika V. A., and Friedrich M., “Epidemiological Trends in Skin Mycoses Worldwide,” Mycoses 51 (2008): 2–15. - PubMed
-
- Ameen M., “Epidemiology of Superficial Fungal Infections,” Clinics in Dermatology 28, no. 2 (2010): 197–201. - PubMed
-
- Stewart C. R., Algu L., Kamran R., et al., “Effect of Onychomycosis and Treatment on Patient‐Reported Quality‐Of‐Life Outcomes: A Systematic Review,” Journal of the American Academy of Dermatology 85, no. 5 (2021): 1227–1239. - PubMed
-
- Lipner S. R. and Scher R. K., “Onychomycosis: Treatment and Prevention of Recurrence,” Journal of the American Academy of Dermatology 80, no. 4 (2019): 853–867. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

