Family Screening in Relatives at Risk for Plakophilin-2-Associated Arrhythmogenic Right Ventricular Cardiomyopathy
- PMID: 40525281
- PMCID: PMC12240474
- DOI: 10.1161/CIRCULATIONAHA.125.074058
Family Screening in Relatives at Risk for Plakophilin-2-Associated Arrhythmogenic Right Ventricular Cardiomyopathy
Abstract
Background: Penetrance and risk of ventricular arrhythmias (VAs) in arrhythmogenic right ventricular cardiomyopathy (ARVC) are increasingly recognized as being genotype specific. Therefore, genotype-informed family screening protocols may lead to safer and more personalized recommendations than the current one-size-fits-all screening recommendations. We aimed to develop a safe, evidence-based plakophilin-2 (PKP2)-specific longitudinal screening algorithm.
Methods: We included 295 relatives (41% male; age 30.9 years [18.0-47.7 years]) with a pathogenic or likely pathogenic PKP2 variant from 145 families. Phenotype was ascertained with ECG, Holter monitoring, and cardiac imaging and classified by the 2010 Task Force Criteria. VA was defined as a composite of sudden cardiac arrest or death, spontaneous sustained ventricular tachycardia, ventricular fibrillation, or appropriate implantable cardioverter defibrillator intervention. We performed Cox regression to determine predictors of ARVC development and multistate modeling to assess the probability of ARVC development and occurrence of VA.
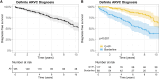
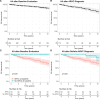
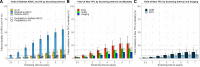
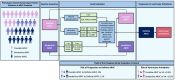
Results: At baseline, 110 relatives (37%) had definite ARVC. During 8.5 years (4.2-12.9 years) of follow-up, 62 of 185 relatives (34%) without definite ARVC at baseline progressed to definite ARVC diagnosis, and 35 of 295 of all relatives (12%) had VA. VAs occurred only in relatives who previously fulfilled definite ARVC diagnosis. Relatives with borderline ARVC (fulfillment of one minor criterion plus the major family history criterion) progressed 5 times faster in the multistate model to definite ARVC diagnosis and compared with genotype-positive/phenotype-negative (G+/P-) relatives (ie, major family history criterion alone). Relatives 20 to 40 years of age had increased risk for developing definite ARVC (hazard ratio, 2.23; P=0.012) compared with those ≥40 years of age. New Task Force Criteria fulfillment most commonly occurred first on ECGs, followed by Holter monitoring and cardiac imaging. Consequently, 3 risk profiles were identified, and appropriate screening protocols were derived: relatives with borderline ARVC (annual ECG and Holter monitoring; complete evaluation [ie, ECGs, Holter monitoring, and imaging] every 2 years), younger (<40 years of age) or symptomatic G+/P- relatives (every 2 years an ECG and Holter monitoring; complete evaluation every 4 years), and older (≥40 years of age) and asymptomatic G+/P- relatives (complete evaluation every 5 years).
Conclusions: An evidence-based longitudinal screening algorithm that integrates age, symptoms, and baseline clinical phenotype may improve patient care and improve efficiency of clinical resource allocation.
Keywords: cardiomyopathies; death, sudden, cardiac; genetics; genotype; precision medicine.
Conflict of interest statement
Dr James has received research support from Stride Bio Inc, Lexeo Therapeutics, and ARVADA Therapeutics. C. Tichnell has received salary support on these grants. Dr te Riele is a consultant for Tenaya, Rocket Pharmaceuticals, and BioMarin for unrelated work (gene therapy trials). Drs James and Gasperetti have served as compensated consultants for LEXEO Therapeutics for unrelated work. Dr Yap has received honoraria (speaker or consultancy fees) from Boston Scientific, Medtronic, Biotronik, and Acutus Medical. In addition, he has received research grants from Medtronic, Biotronik, and Boston Scientific. The other authors report no conflicts.
Figures


References
-
- Bhonsale A, Groeneweg JA, James CA, Dooijes D, Tichnell C, Jongbloed JDH, Murray B, te Riele ASJM, van den Berg MP, Bikker H, et al. Impact of genotype on clinical course in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated mutation carriers. Eur Heart J. 2015;36:847–855. doi: 10.1093/eurheartj/ehu509 - PubMed
-
- Marcus FI, Fontaine GH, Guiraudon G, Frank R, Laurenceau JL, Malergue C, Grosgogeat Y. Right ventricular dysplasia: a report of 24 adult cases. Circulation. 1982;65:384–398. doi: 10.1161/01.cir.65.2.384 - PubMed
-
- Towbin JA, McKenna WJ, Abrams DJ, Ackerman MJ, Calkins H, Darrieux FCC, Daubert JP, de Chillou C, DePasquale EC, Desai MY, et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm. 2019;16:e301–e372. doi: 10.1016/j.hrthm.2019.05.007 - PubMed
-
- Corrado D, Wichter T, Link MS, Hauer RNW, Marchlinski FE, Anastasakis A, Bauce B, Basso C, Brunckhorst C, Tsatsopoulou A, et al. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: an international task force consensus statement. Circulation. 2015;132:441–453. doi: 10.1161/CIRCULATIONAHA.115.017944 - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

