The Non-Coding Regulatory Variant rs2863002 at chr11p11.2 Increases Neuroblastoma Risk by Affecting HSD17B12 Expression and Lipid Metabolism
- PMID: 40525640
- PMCID: PMC12412506
- DOI: 10.1002/advs.202415181
The Non-Coding Regulatory Variant rs2863002 at chr11p11.2 Increases Neuroblastoma Risk by Affecting HSD17B12 Expression and Lipid Metabolism
Abstract
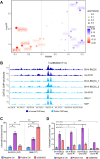
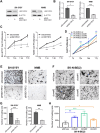
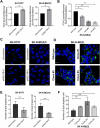
A Genome-wide association study (GWAS) on a European-American cohort identified chr11p11.2 as a neuroblastoma predisposition locus. Combining in-house and public genomic data from neuroblastoma cell lines, this work implicates rs2863002 as the candidate causal variant at the 11p11.2 locus, confirming its cis-regulatory activity through a luciferase reporter assay. The genetic association of rs2863002 with neuroblastoma risk is validated in an Italian case-control cohort. Using ChIP-qPCR, Hi-C, and CRISPR genome editing, this work deciphers the regulatory mechanisms at the risk locus, demonstrating that the rs2863002-C risk allele regulates HSD17B12 expression and reduces GATA3 binding affinity. In vitro functional assays and targeted lipidomic analyses reveal the involvement of the rs2863002-C risk allele in tumorigenicity and modulation of lipid metabolism in neuroblastoma cells through HSD17B12 regulation. This study provides new insights into the genetic basis of neuroblastoma and underscores the importance of post-GWAS functional characterization of risk loci in uncovering relevant biological findings for understanding complex diseases.
Keywords: HSD17B12; GWAS; SNP; functional genomics; genetic predisposition; lipid metabolism; neuroblastoma.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Matthay K. K., Maris J. M., Schleiermacher G., Nakagawara A., Mackall C. L., Diller L., Weiss W. A., Nat. Rev. Dis. Primers 2016, 2, 16078. - PubMed
-
- Kim J., Vaksman Z., Egolf L. E., Kaufman R., Evans J. P., Conkrite K. L., Danesh A., Lopez G., Randall M. P., Dent M. H., Farra L. M., Menghani N. L., Dymek M., Desai H., Hausler R., Hicks B., Auvil J. G., Gerhard D. S., Hakonarson H., Maxwell K. N., Cole K. A., Pugh T. J., Bosse K. R., Khan J., Wei J. S., Maris J. M., Stewart D. R., Diskin S. J., J. Natl. Cancer Inst. 2024, 116, 149. - PMC - PubMed
MeSH terms
Substances
Grants and funding
- R01 CA237562/CA/NCI NIH HHS/United States
- OPEN Associazione Oncologia Pediatrica e Neuroblastoma ONLUS
- R01 CA124709/CA/NCI NIH HHS/United States
- R35 CA220500/CA/NCI NIH HHS/United States
- PRIN P2022NFCPM/Italian Ministry for University and Research (MUR) National Recovery and Resilience Plan (PNRR)
- 25 796/Associazione Italiana per la Ricerca sul Cancro
- R35-CA220500/NH/NIH HHS/United States
- Fondazione Italiana per la Lotta al Neuroblastoma
- R01-CA237562/NH/NIH HHS/United States
- R35-CA124709/NH/NIH HHS/United States
- CUP-E53D23015470001/Italian Ministry for University and Research (MUR) National Recovery and Resilience Plan (PNRR)
LinkOut - more resources
Full Text Sources
Medical
